当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Dipolar Cycloaddition Reaction To Access 6-Methyl-4,5,6,7-tetrahydro-1H-[1,2,3]triazolo[4,5-c]pyridines Enables the Discovery Synthesis and Preclinical Profiling of a P2X7 Antagonist Clinical Candidate
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01279 Christa C. Chrovian 1 , Akinola Soyode-Johnson 1 , Alexander A. Peterson 1 , Christine F. Gelin 1 , Xiaohu Deng 1 , Curt A. Dvorak 1 , Nicholas I. Carruthers 1 , Brian Lord 1 , Ian Fraser 1 , Leah Aluisio 1 , Kevin J. Coe 1 , Brian Scott 1 , Tatiana Koudriakova 1 , Freddy Schoetens 1 , Kia Sepassi 1 , David J. Gallacher 2 , Anindya Bhattacharya 1 , Michael A. Letavic 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jmedchem.7b01279 Christa C. Chrovian 1 , Akinola Soyode-Johnson 1 , Alexander A. Peterson 1 , Christine F. Gelin 1 , Xiaohu Deng 1 , Curt A. Dvorak 1 , Nicholas I. Carruthers 1 , Brian Lord 1 , Ian Fraser 1 , Leah Aluisio 1 , Kevin J. Coe 1 , Brian Scott 1 , Tatiana Koudriakova 1 , Freddy Schoetens 1 , Kia Sepassi 1 , David J. Gallacher 2 , Anindya Bhattacharya 1 , Michael A. Letavic 1
Affiliation
|
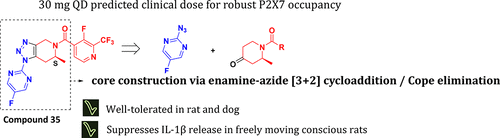
A single pot dipolar cycloaddition reaction/Cope elimination sequence was developed to access novel 1,4,6,7-tetrahydro-5H-[1,2,3]triazolo[4,5-c]pyridine P2X7 antagonists that contain a synthetically challenging chiral center. The structure–activity relationships of the new compounds are described. Two of these compounds, (S)-(2-fluoro-3-(trifluoromethyl)phenyl)(1-(5-fluoropyrimidin-2-yl)-6-methyl-1,4,6,7-tetrahydro-5H-[1,2,3]triazolo[4,5-c]pyridin-5-yl)methanone (compound 29) and (S)-(3-fluoro-2-(trifluoromethyl)pyridin-4-yl)(1-(5-fluoropyrimidin-2-yl)-6-methyl-1,4,6,7-tetrahydro-5H-[1,2,3]triazolo[4,5-c]pyridin-5-yl)methanone (compound 35), were found to have robust P2X7 receptor occupancy at low doses in rat with ED50 values of 0.06 and 0.07 mg/kg, respectively. Compound 35 had notable solubility compared to 29 and showed good tolerability in preclinical species. Compound 35 was chosen as a clinical candidate for advancement into phase I clinical trials to assess safety and tolerability in healthy human subjects prior to the initiation of proof of concept studies for the treatment of mood disorders.
中文翻译:

通过偶极环加成反应获得6-甲基-4,5,6,7-四氢-1 H- [1,2,3]三唑并[4,5- c ]吡啶可实现P2X7拮抗剂的发现合成和临床前分析。临床候选人
开发了一个单锅偶极环加成反应/ Cope消除序列,以访问新颖的1,4,6,7-四氢-5 H- [1,2,3]三唑[4,5- c ]吡啶P2X7拮抗剂,该拮抗剂包含合成的具有挑战性的手性中心。描述了新化合物的结构-活性关系。这些化合物中的两种,(S)-(2-氟-3-(三氟甲基)苯基)(1-(5-氟嘧啶-2-基)-6-甲基-1,4,6,7-四氢-5 H -[1,2,3]三唑并[4,5 - c ]吡啶-5-基)甲酮(化合物29)和(S)-(3-氟-2-(三氟甲基)吡啶-4-基)(1 -(5-氟嘧啶-2-基)-6-甲基-1,4,6,7-四氢-5 H- [1,2,3]三唑[4,5- c在低剂量下,发现大鼠吡啶[5-吡啶基]甲酮(化合物35)具有很强的P2X7受体占有率,ED 50值分别为0.06和0.07 mg / kg。与29相比,化合物35的溶解度显着,并且对临床前物种显示出良好的耐受性。选择化合物35作为进入I期临床试验的临床候选药物,以评估健康人受试者在治疗情绪障碍的概念验证研究之前的安全性和耐受性。
更新日期:2017-12-20
中文翻译:

通过偶极环加成反应获得6-甲基-4,5,6,7-四氢-1 H- [1,2,3]三唑并[4,5- c ]吡啶可实现P2X7拮抗剂的发现合成和临床前分析。临床候选人
开发了一个单锅偶极环加成反应/ Cope消除序列,以访问新颖的1,4,6,7-四氢-5 H- [1,2,3]三唑[4,5- c ]吡啶P2X7拮抗剂,该拮抗剂包含合成的具有挑战性的手性中心。描述了新化合物的结构-活性关系。这些化合物中的两种,(S)-(2-氟-3-(三氟甲基)苯基)(1-(5-氟嘧啶-2-基)-6-甲基-1,4,6,7-四氢-5 H -[1,2,3]三唑并[4,5 - c ]吡啶-5-基)甲酮(化合物29)和(S)-(3-氟-2-(三氟甲基)吡啶-4-基)(1 -(5-氟嘧啶-2-基)-6-甲基-1,4,6,7-四氢-5 H- [1,2,3]三唑[4,5- c在低剂量下,发现大鼠吡啶[5-吡啶基]甲酮(化合物35)具有很强的P2X7受体占有率,ED 50值分别为0.06和0.07 mg / kg。与29相比,化合物35的溶解度显着,并且对临床前物种显示出良好的耐受性。选择化合物35作为进入I期临床试验的临床候选药物,以评估健康人受试者在治疗情绪障碍的概念验证研究之前的安全性和耐受性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号