当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Multisubstituted Biaryl Skeleton by Chiral Phosphoric Acid Catalyzed Desymmetrization/Kinetic Resolution Sequence
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-02-26 , DOI: 10.1021/ja311902f Keiji Mori 1 , Yuki Ichikawa 1 , Manato Kobayashi 1 , Yukihiro Shibata 2 , Masahiro Yamanaka 2 , Takahiko Akiyama 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2013-02-26 , DOI: 10.1021/ja311902f Keiji Mori 1 , Yuki Ichikawa 1 , Manato Kobayashi 1 , Yukihiro Shibata 2 , Masahiro Yamanaka 2 , Takahiko Akiyama 1
Affiliation
|
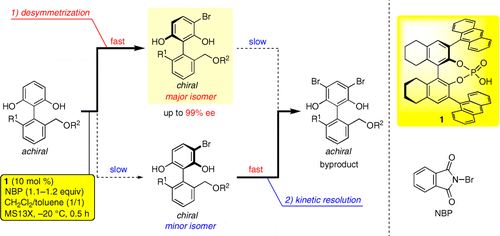
Described herein is the enantioselective synthesis of multisubstituted biaryl derivatives by chiral phosphoric acid catalyzed asymmetric bromination. Two asymmetric reactions (desymmetrization and kinetic resolution) proceeded successively to afford chiral biaryls in excellent enantioselectivities (up to 99% ee). Both experimental and computational studies suggested that this excellent selectivity could be achieved via a highly organized hydrogen bond network among a substrate, a catalyst (chiral phosphoric acid), and a brominating reagent (N-bromophthalimide).
中文翻译:

手性磷酸催化去对称化/动力学拆分序列对映选择性合成多取代联芳基骨架
本文描述了通过手性磷酸催化的不对称溴化作用对多取代联芳基衍生物的对映选择性合成。两个不对称反应(去对称化和动力学拆分)相继进行,以优异的对映选择性(高达 99% ee)得到手性联芳基化合物。实验和计算研究都表明,这种优异的选择性可以通过底物、催化剂(手性磷酸)和溴化试剂(N-溴邻苯二甲酰亚胺)之间高度有序的氢键网络实现。
更新日期:2013-02-26
中文翻译:

手性磷酸催化去对称化/动力学拆分序列对映选择性合成多取代联芳基骨架
本文描述了通过手性磷酸催化的不对称溴化作用对多取代联芳基衍生物的对映选择性合成。两个不对称反应(去对称化和动力学拆分)相继进行,以优异的对映选择性(高达 99% ee)得到手性联芳基化合物。实验和计算研究都表明,这种优异的选择性可以通过底物、催化剂(手性磷酸)和溴化试剂(N-溴邻苯二甲酰亚胺)之间高度有序的氢键网络实现。































 京公网安备 11010802027423号
京公网安备 11010802027423号