当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Active-Site Engineering of Benzaldehyde Lyase Shows That a Point Mutation Can Confer Both New Reactivity and Susceptibility to Mechanism-Based Inhibition
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-01-20 , DOI: 10.1021/ja907064w Gabriel S. Brandt 1 , Malea M. Kneen 1 , Gregory A. Petsko 1 , Dagmar Ringe 1 , Michael J. McLeish 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2010-01-20 , DOI: 10.1021/ja907064w Gabriel S. Brandt 1 , Malea M. Kneen 1 , Gregory A. Petsko 1 , Dagmar Ringe 1 , Michael J. McLeish 1
Affiliation
|
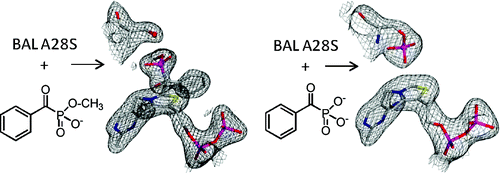
Benzaldehyde lyase (BAL) from Pseudomonas putida is a thiamin diphosphate (ThDP)-dependent enzyme that catalyzes the breakdown of (R)-benzoin. Here we report that a point mutant, BAL A28S, not only catalyzes the decarboxylation of benzoylformate but, like benzoylformate decarboxylase (BFDC), is also inactivated by the benzoylformate analogues methyl benzoylphosphonate (MBP) and benzoylphosphonate (BP). The latter has no effect on wild-type BAL, and the inactivation of the A28S variant is shown to result from phosphorylation of the newly introduced serine residue. This lends support to the proposal that an appropriately placed nucleophile facilitates the expulsion of carbon dioxide from the active site in many ThDP-dependent decarboxylases.
中文翻译:

苯甲醛裂解酶的活性位点工程表明点突变可以赋予基于机制的抑制新的反应性和敏感性
来自恶臭假单胞菌的苯甲醛裂解酶 (BAL) 是一种硫胺二磷酸 (ThDP) 依赖性酶,可催化 (R)-安息香的分解。在这里,我们报告了一个点突变体 BAL A28S,它不仅催化苯甲酰甲酸酯的脱羧,而且像苯甲酰甲酸酯脱羧酶 (BFDC) 一样,也被苯甲酰甲酸酯类似物苯甲酰膦酸甲酯 (MBP) 和苯甲酰膦酸酯 (BP) 灭活。后者对野生型 BAL 没有影响,并且 A28S 变体的失活是由新引入的丝氨酸残基的磷酸化引起的。这为以下提议提供支持,即适当放置的亲核试剂促进二氧化碳从许多 ThDP 依赖性脱羧酶的活性位点排出。
更新日期:2010-01-20
中文翻译:

苯甲醛裂解酶的活性位点工程表明点突变可以赋予基于机制的抑制新的反应性和敏感性
来自恶臭假单胞菌的苯甲醛裂解酶 (BAL) 是一种硫胺二磷酸 (ThDP) 依赖性酶,可催化 (R)-安息香的分解。在这里,我们报告了一个点突变体 BAL A28S,它不仅催化苯甲酰甲酸酯的脱羧,而且像苯甲酰甲酸酯脱羧酶 (BFDC) 一样,也被苯甲酰甲酸酯类似物苯甲酰膦酸甲酯 (MBP) 和苯甲酰膦酸酯 (BP) 灭活。后者对野生型 BAL 没有影响,并且 A28S 变体的失活是由新引入的丝氨酸残基的磷酸化引起的。这为以下提议提供支持,即适当放置的亲核试剂促进二氧化碳从许多 ThDP 依赖性脱羧酶的活性位点排出。

















































 京公网安备 11010802027423号
京公网安备 11010802027423号