当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Palladium-Catalyzed Decarboxylative Asymmetric Allylic Alkylation of Enol Carbonates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-12-30 , DOI: 10.1021/ja9053948 Barry M Trost 1 , Jiayi Xu , Thomas Schmidt
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-12-30 , DOI: 10.1021/ja9053948 Barry M Trost 1 , Jiayi Xu , Thomas Schmidt
Affiliation
|
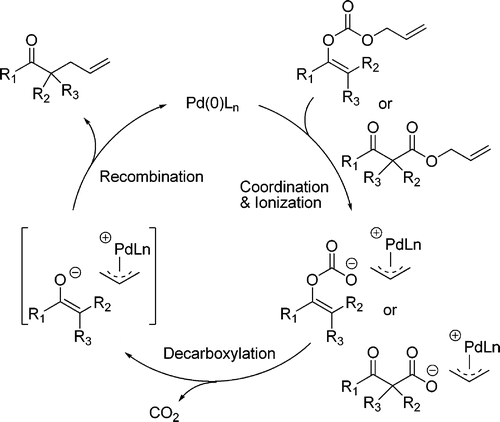
Palladium-catalyzed decarboxylative asymmetric allylic alkylation (DAAA) of allyl enol carbonates as a highly chemo-, regio-, and enantioselective process for the synthesis of ketones bearing either a quaternary or a tertiary alpha-stereogenic center has been investigated in detail. Chiral ligand L4 was found to be optimal in the DAAA of a broad scope of cyclic and acyclic ketones including simple aliphatic ketones with more than one enolizable proton. The allyl moiety of the carbonates has been extended to a variety of cyclic or acyclic disubstituted allyl groups. Our mechanistic studies reveal that, similar to the direct allylation of lithium enolates, the DAAA reaction proceeds through an "outer sphere" S(N)2 type of attack on the pi-allylpalladium complex by the enolate. An important difference between the DAAA reaction and the direct allylation of lithium enolates is that in the DAAA reaction, the nucleophile and the electrophile were generated simultaneously. Since the pi-allylpalladium cation must serve as the counterion for the enolate, the enolate probably exists as a tight-ion-pair. This largely prevents the common side reactions of enolates associated with the equilibrium between different enolates. The much milder reaction conditions as well as the much broader substrate scope also represent the advantages of the DAAA reaction over the direct allylation of preformed metal enolates.
中文翻译:

钯催化烯醇碳酸酯的脱羧不对称烯丙基烷基化
钯催化的烯丙基烯醇碳酸酯的脱羧不对称烯丙基烷基化 (DAAA) 作为一种高度化学、区域和对映选择性的过程,用于合成带有四级或三级 α 立体中心的酮,已进行了详细研究。发现手性配体 L4 在多种环状和无环酮(包括具有多个烯醇化质子的简单脂肪族酮)的 DAAA 中是最佳的。碳酸酯的烯丙基部分已扩展到各种环状或无环二取代的烯丙基。我们的机理研究表明,与烯醇化锂的直接烯丙基化类似,DAAA 反应是通过烯醇化物对 pi-烯丙基钯配合物的“外层”S(N)2 类型的攻击来进行的。 DAAA反应与烯醇锂直接烯丙基化之间的一个重要区别在于,在DAAA反应中,亲核试剂和亲电试剂同时产生。由于π-烯丙基钯阳离子必须充当烯醇化物的抗衡离子,因此烯醇化物可能以紧密离子对的形式存在。这在很大程度上防止了与不同烯醇化物之间的平衡相关的常见烯醇化物副反应。更温和的反应条件以及更广泛的底物范围也代表了 DAAA 反应相对于预先形成的金属烯醇化物的直接烯丙基化的优势。
更新日期:2009-12-30
中文翻译:

钯催化烯醇碳酸酯的脱羧不对称烯丙基烷基化
钯催化的烯丙基烯醇碳酸酯的脱羧不对称烯丙基烷基化 (DAAA) 作为一种高度化学、区域和对映选择性的过程,用于合成带有四级或三级 α 立体中心的酮,已进行了详细研究。发现手性配体 L4 在多种环状和无环酮(包括具有多个烯醇化质子的简单脂肪族酮)的 DAAA 中是最佳的。碳酸酯的烯丙基部分已扩展到各种环状或无环二取代的烯丙基。我们的机理研究表明,与烯醇化锂的直接烯丙基化类似,DAAA 反应是通过烯醇化物对 pi-烯丙基钯配合物的“外层”S(N)2 类型的攻击来进行的。 DAAA反应与烯醇锂直接烯丙基化之间的一个重要区别在于,在DAAA反应中,亲核试剂和亲电试剂同时产生。由于π-烯丙基钯阳离子必须充当烯醇化物的抗衡离子,因此烯醇化物可能以紧密离子对的形式存在。这在很大程度上防止了与不同烯醇化物之间的平衡相关的常见烯醇化物副反应。更温和的反应条件以及更广泛的底物范围也代表了 DAAA 反应相对于预先形成的金属烯醇化物的直接烯丙基化的优势。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号