当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvation Structure of Sodium Bis(fluorosulfonyl)imide-Glyme Solvate Ionic Liquids and Its Influence on Cycling of Na-MNC Cathodes
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jpcb.7b10158
Pieter Geysens 1 , Vijay Shankar Rangasamy 2 , Savitha Thayumanasundaram 2 , Koen Robeyns 3 , Luc Van Meervelt 1 , Jean-Pierre Locquet 2 , Jan Fransaer 4 , Koen Binnemans 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-12-20 00:00:00 , DOI: 10.1021/acs.jpcb.7b10158
Pieter Geysens 1 , Vijay Shankar Rangasamy 2 , Savitha Thayumanasundaram 2 , Koen Robeyns 3 , Luc Van Meervelt 1 , Jean-Pierre Locquet 2 , Jan Fransaer 4 , Koen Binnemans 1
Affiliation
|
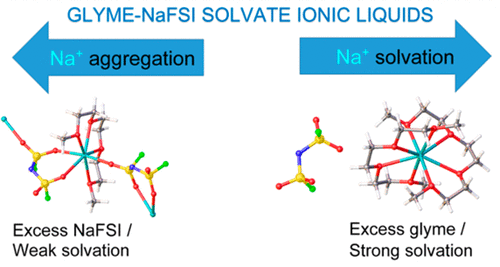
Electrolytes consisting of sodium bis(fluorosulfonyl)imide (NaFSI) dissolved in glymes (monoglyme, diglyme, and triglyme) were characterized by FT-Raman spectroscopy and 13C, 17O, and 23Na NMR spectroscopy. The glyme:NaFSI molar ratio was varied from 50:1 to 1:1, and it was observed that, in the dilute electrolytes, the sodium salt is completely dissociated into solvent separated ion pairs (SSIPs). However, contact ion pairs (CIPs) and aggregates (AGGs) become the predominant species in more concentrated solutions. Some of the electrolytes with the highest concentrations can be classified as solvate ionic liquids (SILs), where all of the solvent molecules are coordinated to sodium cations. Therefore, these electrolytes are fundamentally different from more dilute electrolytes which are typically used in commercially available secondary batteries. The melting point or glass transition temperature, dynamic viscosity, density, sodium concentration, and ionic conductivity of these solvate ionic liquids are reported as well as the crystal structures of [Na(G3)][FSI] and [Na(G3)2][FSI]. Galvanostatic cycling experiments were performed in coin-type cells with a Na2/3[Mn0.55Ni0.30Co0.15]O2 cathode to study the influence of these electrolytes on the electrochemical stability and charge/discharge behavior.
中文翻译:

双(氟磺酰基)酰亚胺-甘氨酸钠离子液体的溶剂化结构及其对Na-MNC阴极循环的影响
通过FT-拉曼光谱和13 C,17 O和23表征由溶解在二甘醇二甲醚(二甘醇二甲醚,二甘醇二甲醚和三甘醇二甲醚)中的双(氟磺酰基)酰亚胺钠(NaFSI)组成的电解质。Na NMR光谱。甘醇二甲醚:NaFSI摩尔比从50:1到1:1不等,并且观察到,在稀电解质中,钠盐完全分解成溶剂分离的离子对(SSIP)。但是,在更浓缩的溶液中,接触离子对(CIP)和聚集体(AGG)成为主要种类。某些浓度最高的电解质可归类为溶剂化物离子液体(SILs),其中所有溶剂分子均与钠阳离子配位。因此,这些电解质与通常在市售二次电池中使用的更稀薄的电解质根本上不同。熔点或玻璃化转变温度,动态粘度,密度,钠浓度,2 ] [FSI]。在具有Na 2/3 [Mn 0.55 Ni 0.30 Co 0.15 ] O 2阴极的硬币型电池中进行恒电流循环实验,以研究这些电解质对电化学稳定性和充电/放电行为的影响。
更新日期:2017-12-20
中文翻译:

双(氟磺酰基)酰亚胺-甘氨酸钠离子液体的溶剂化结构及其对Na-MNC阴极循环的影响
通过FT-拉曼光谱和13 C,17 O和23表征由溶解在二甘醇二甲醚(二甘醇二甲醚,二甘醇二甲醚和三甘醇二甲醚)中的双(氟磺酰基)酰亚胺钠(NaFSI)组成的电解质。Na NMR光谱。甘醇二甲醚:NaFSI摩尔比从50:1到1:1不等,并且观察到,在稀电解质中,钠盐完全分解成溶剂分离的离子对(SSIP)。但是,在更浓缩的溶液中,接触离子对(CIP)和聚集体(AGG)成为主要种类。某些浓度最高的电解质可归类为溶剂化物离子液体(SILs),其中所有溶剂分子均与钠阳离子配位。因此,这些电解质与通常在市售二次电池中使用的更稀薄的电解质根本上不同。熔点或玻璃化转变温度,动态粘度,密度,钠浓度,2 ] [FSI]。在具有Na 2/3 [Mn 0.55 Ni 0.30 Co 0.15 ] O 2阴极的硬币型电池中进行恒电流循环实验,以研究这些电解质对电化学稳定性和充电/放电行为的影响。






































 京公网安备 11010802027423号
京公网安备 11010802027423号