当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Scope and Mechanism of Intramolecular Aziridination of Cyclopent-3-enyl-methylamines to 1-Azatricyclo[2.2.1.02,6]heptanes with Lead Tetraacetate
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-26 , DOI: 10.1021/ja9044136 Huayou Hu 1 , Juan A Faraldos , Robert M Coates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2009-08-26 , DOI: 10.1021/ja9044136 Huayou Hu 1 , Juan A Faraldos , Robert M Coates
Affiliation
|
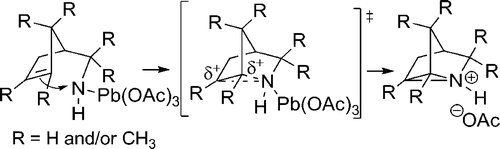
A series of seven cyclopent-3-en-1-ylmethylamines bearing one, two, or three methyl substituents at the C2, C3, C4, or C(alpha) positions, including the unsubstituted parent, was accessed by ring-closing metatheses of alpha,alpha-diallylacetonitrile (or methallyl variants) and alpha,alpha-diallylacetone followed by hydride reductions or reductive amination, or by Curtius degradations of alpha,alpha-dimethyl- and 2,2,3-trimethylcyclopent-3-enylacetic acids. Oxidation of the primary amines with Pb(OAc)(4) in CH(2)Cl(2), CHCl(3) or benzene in the presence of K(2)CO(3) effected efficient intramolecular aziridinations, in all cases except the alpha-methyl analogue (16), to form the corresponding 1-azatricyclo[2.2.1.0(2,6)]heptanes, including the novel monoterpene analogues, 1-azatricyclene and the 2-azatricyclene enantiomers. The cumulative rate increases of aziridination reactions observed by (1)H NMR spectroscopy in CDCl(3) resulting from the presence of one or two methyl groups on the cyclopentene double bond, in comparison to the rate of the unsubstituted parent amine (1:17.5:>280), indicate a highly electrophilic intermediate as the nitrene donor and a symmetrical aziridine-like transition state. A mechanism is outlined in which the amine displaces an acetate ligand from Pb(OAc)(4) to form a lead(IV) amide intermediate RNHPb(OAc)(3) proposed as the actual aziridinating species.
中文翻译:

四乙酸铅分子内氮丙啶化环戊-3-烯基甲胺生成1-氮杂三环[2.2.1.02,6]庚烷的范围和机理
通过闭环复分解获得了一系列在 C2、C3、C4 或 C(α) 位(包括未取代的母体)带有一个、两个或三个甲基取代基的七个环戊-3-en-1-基甲基胺α,α-二烯丙基乙腈(或甲代烯丙基变体)和α,α-二烯丙基丙酮,然后进行氢化物还原或还原胺化,或通过α,α-二甲基-和2,2,3-三甲基环戊-3-烯基乙酸的Curtius降解。在 K(2)CO(3) 存在下,在 CH(2)Cl(2)、CHCl(3) 或苯中用 Pb(OAc)(4) 氧化伯胺可实现有效的分子内氮丙啶化,除了α-甲基类似物(16),形成相应的1-氮杂三环[2.2.1.0(2,6)]庚烷,包括新型单萜类似物、1-氮杂三环烯和2-氮杂三环烯对映体。与未取代的母体胺的速率相比,由于环戊烯双键上存在一个或两个甲基,通过 (1)H NMR 光谱在 CDCl(3) 中观察到氮丙啶化反应的累积速率增加 (1:17.5) :>280),表示作为氮宾供体的高度亲电中间体和对称的氮丙啶样过渡态。概述了一种机制,其中胺取代 Pb(OAc)(4) 中的乙酸酯配体,形成铅 (IV) 酰胺中间体 RNHPb(OAc)(3),建议作为实际的氮丙啶化物质。
更新日期:2009-08-26
中文翻译:

四乙酸铅分子内氮丙啶化环戊-3-烯基甲胺生成1-氮杂三环[2.2.1.02,6]庚烷的范围和机理
通过闭环复分解获得了一系列在 C2、C3、C4 或 C(α) 位(包括未取代的母体)带有一个、两个或三个甲基取代基的七个环戊-3-en-1-基甲基胺α,α-二烯丙基乙腈(或甲代烯丙基变体)和α,α-二烯丙基丙酮,然后进行氢化物还原或还原胺化,或通过α,α-二甲基-和2,2,3-三甲基环戊-3-烯基乙酸的Curtius降解。在 K(2)CO(3) 存在下,在 CH(2)Cl(2)、CHCl(3) 或苯中用 Pb(OAc)(4) 氧化伯胺可实现有效的分子内氮丙啶化,除了α-甲基类似物(16),形成相应的1-氮杂三环[2.2.1.0(2,6)]庚烷,包括新型单萜类似物、1-氮杂三环烯和2-氮杂三环烯对映体。与未取代的母体胺的速率相比,由于环戊烯双键上存在一个或两个甲基,通过 (1)H NMR 光谱在 CDCl(3) 中观察到氮丙啶化反应的累积速率增加 (1:17.5) :>280),表示作为氮宾供体的高度亲电中间体和对称的氮丙啶样过渡态。概述了一种机制,其中胺取代 Pb(OAc)(4) 中的乙酸酯配体,形成铅 (IV) 酰胺中间体 RNHPb(OAc)(3),建议作为实际的氮丙啶化物质。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号