当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bicyclization of Azomethine Ylide: Access to Highly Functionalized 3H-Pyrrolo[2,3-c]quinolines
Organic Letters ( IF 4.9 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.orglett.7b03434 Yang Men 1 , Jinhuan Dong 2 , Shan Wang 1 , Xianxiu Xu 1, 2
Organic Letters ( IF 4.9 ) Pub Date : 2017-12-05 00:00:00 , DOI: 10.1021/acs.orglett.7b03434 Yang Men 1 , Jinhuan Dong 2 , Shan Wang 1 , Xianxiu Xu 1, 2
Affiliation
|
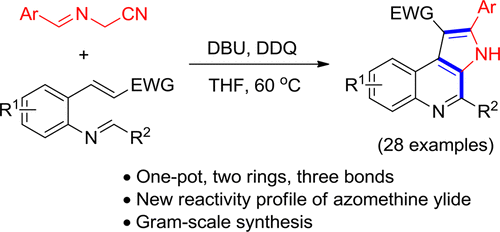
A tandem bicyclization of azomethine ylides with methyleneaminochalcones was developed for the straightforward and facile synthesis of 2-substituted polyfunctionalized pyrrolo[2,3-c]quinolines. Both an unusual reactivity profile of azomethine ylide and a novel strategy for the construction of the tricyclic framework by the successive construction of the pyridine and pyrrole rings were exhibited in this domino reaction. Two intermediates are isolated in the control experiments, and thus a tandem bicyclization/elimination/oxidative aromatization process is proposed for the reaction mechanism.
中文翻译:

偶氮甲Y素内酯的双环化:获得高度官能化的3 H-吡咯并[2,3- c ]喹啉
已开发了亚甲基氨基查耳酮与甲亚胺基亚胺串联双环化反应的方法,可用于简单,方便地合成2-取代的多官能化吡咯并[2,3- c ]喹啉。在该多米诺反应中,既显示出偶氮甲meth叶立德的异常反应谱,又显示了通过连续构建吡啶和吡咯环来构建三环骨架的新策略。在对照实验中分离出两种中间体,因此提出了串联双环化/消除/氧化芳构化的反应机理。
更新日期:2017-12-05
中文翻译:

偶氮甲Y素内酯的双环化:获得高度官能化的3 H-吡咯并[2,3- c ]喹啉
已开发了亚甲基氨基查耳酮与甲亚胺基亚胺串联双环化反应的方法,可用于简单,方便地合成2-取代的多官能化吡咯并[2,3- c ]喹啉。在该多米诺反应中,既显示出偶氮甲meth叶立德的异常反应谱,又显示了通过连续构建吡啶和吡咯环来构建三环骨架的新策略。在对照实验中分离出两种中间体,因此提出了串联双环化/消除/氧化芳构化的反应机理。






























 京公网安备 11010802027423号
京公网安备 11010802027423号