当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Reversible Insertion of a C=C Bond into Magnesium(I) Dimers: Generation of Highly Active 1,2-Dimagnesioethane Compounds
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-08 , DOI: 10.1021/jacs.7b11368 Aaron J. Boutland 1 , Ashlea Carroll 1 , Carlos Alvarez Lamsfus 2 , Andreas Stasch 1 , Laurent Maron 2 , Cameron Jones 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-08 , DOI: 10.1021/jacs.7b11368 Aaron J. Boutland 1 , Ashlea Carroll 1 , Carlos Alvarez Lamsfus 2 , Andreas Stasch 1 , Laurent Maron 2 , Cameron Jones 1
Affiliation
|
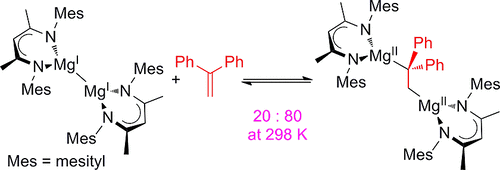
The insertion of 1,1-diphenylethylene into the Mg-Mg bond of two magnesium(I) dimers, [(ArNacnac)Mg-]2 (Ar = C6H2Me3-2,4,6 (Mes); C6H3Et2-2,6 (Dep)), yielding 1,2-dimagnesioethane products, [{(ArNacnac)Mg}2(μ-CH2CPh2)], is described. These reactions are readily reversible at room temperature and thus represent the first examples of room-temperature reversible redox processes for s-block metal complexes. The 1,2-dimagnesioethane products are highly activated magnesium alkyls and show unprecedented, uncatalyzed reactivity toward H2, CO, and ethylene. Computational studies have investigated the mechanisms of all presented reaction types.
中文翻译:

C=C 键可逆插入镁 (I) 二聚体:生成高活性 1,2-二氧化镁乙烷化合物
1,1-二苯基乙烯插入到两个镁(I)二聚体的 Mg-Mg 键中,[(ArNacnac)Mg-]2 (Ar = C6H2Me3-2,4,6 (Mes); C6H3Et2-2,6 ( Dep)),描述了产生 1,2-二氧化镁乙烷产品 [{(ArNacnac)Mg}2(μ-CH2CPh2)]。这些反应在室温下很容易可逆,因此代表了 s 区金属配合物的室温可逆氧化还原过程的第一个例子。1,2-二氧化镁乙烷产品是高度活化的烷基镁,对 H2、CO 和乙烯表现出前所未有的未催化反应性。计算研究已经调查了所有呈现的反应类型的机制。
更新日期:2017-12-08
中文翻译:

C=C 键可逆插入镁 (I) 二聚体:生成高活性 1,2-二氧化镁乙烷化合物
1,1-二苯基乙烯插入到两个镁(I)二聚体的 Mg-Mg 键中,[(ArNacnac)Mg-]2 (Ar = C6H2Me3-2,4,6 (Mes); C6H3Et2-2,6 ( Dep)),描述了产生 1,2-二氧化镁乙烷产品 [{(ArNacnac)Mg}2(μ-CH2CPh2)]。这些反应在室温下很容易可逆,因此代表了 s 区金属配合物的室温可逆氧化还原过程的第一个例子。1,2-二氧化镁乙烷产品是高度活化的烷基镁,对 H2、CO 和乙烯表现出前所未有的未催化反应性。计算研究已经调查了所有呈现的反应类型的机制。































 京公网安备 11010802027423号
京公网安备 11010802027423号