当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Syntheses, Crystal Structures and Photophysical Aspects of Discrete and Polymeric Azido‐Bridged Zinc(II) and Cadmium(II) Complexes: Sensing Properties and Structural Resemblance
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-12-04 , DOI: 10.1002/slct.201701944 Shuvayan Roy 1 , Sagarika Bhattacharya 1 , Sasankasekhar Mohanta 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-12-04 , DOI: 10.1002/slct.201701944 Shuvayan Roy 1 , Sagarika Bhattacharya 1 , Sasankasekhar Mohanta 1
Affiliation
|
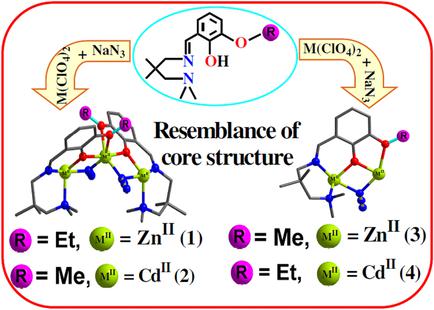
The work in this report presents syntheses, characterization, crystal structures and steady state and time resolved photophysical properties of four compounds of composition [ZnII3L12(N3)4] (1), [CdII3L22(N3)4]n (2), [ZnII2L2(N3)3]n (3) and [CdII2L1(N3)3]n (4), where HL1 and HL2 are the Schiff base ligands, obtained on 1:1 condensation of 3‐ethoxysalicylaldehyde (for HL1)/3‐methoxysalicylaldehyde (for HL2) and N,N,2,2‐tetramethyl‐1,3‐propanediamine. Compound 1 is a discrete trizinc(II) system, while the other three compounds are one‐dimensional coordination polymers, where the building units are, respectively, tricadmium(II), dizinc(II) and dicadmium(II) moieties. The nuclearities of the cores in the ZnII–azido compound from HL1 and the CdII–azido compound from HL2 are similar (1 and 2 are trinuclear) and, similarly, the ZnII–azido compound from HL2 and the CdII–azido compound from HL1 are similar (3 and 4 are dinuclear), revealing an unusual structural resemblance of two metal ions of two different rows in the periodic table as the function of slight change in the ligand periphery (OEt in HL1, OMe in HL2). The steady state/time‐resolved photophysical properties reveal that cadmium(II) enhances slightly but zinc(II) enhances appreciably the fluorescence of the Schiff base ligands HL1 and HL2. It has been found that both HL1 and HL2 are fluorogenic sensors of zinc(II).
中文翻译:

离散和聚合叠氮基桥连锌(II)和镉(II)配合物的合成,晶体结构和光物理方面:传感特性和结构相似性
本报告中的工作介绍了四种成分为[Zn II 3 L 1 2(N 3)4 ](1),[Cd II 3 L 2 2( N 3)4 ] n(2),[Zn II 2 L 2(N 3)3 ] n(3)和[Cd II 2 L 1(N 3))3 ] n(4),其中HL 1和HL 2是席夫碱配体,是通过3-乙氧基水杨醛(对于HL 1)/ 3-甲氧基水杨醛(对于HL 2)和N,N,2 1:1缩合获得的,2-四甲基-1,3-丙二胺。化合物1是离散的Trizinc(II)系统,而其他三种化合物是一维配位聚合物,其建筑单元分别是Tricadmium(II),dizinc(II)和dicadmium(II)部分。HL 1的Zn II-叠氮基化合物和HL的Cd II-叠氮基化合物核的核原子核2是相似的(1和2是三核),类似地,所述锌II从HL -叠氮基化合物2和CD II从HL -叠氮化合物1是相似的(3和4是双核),揭示了两个不同寻常的结构相似在周期表中作为配位体周边略有变化的函数的两个不同的行的金属离子(OET在HL 1,OME在HL 2)。稳态/时间分辨的光物理性质表明,镉(II)略微增强,而锌(II)则显着增强席夫碱配体HL 1和HL的荧光2。已经发现HL 1和HL 2都是锌(II)的荧光传感器。
更新日期:2017-12-04
中文翻译:

离散和聚合叠氮基桥连锌(II)和镉(II)配合物的合成,晶体结构和光物理方面:传感特性和结构相似性
本报告中的工作介绍了四种成分为[Zn II 3 L 1 2(N 3)4 ](1),[Cd II 3 L 2 2( N 3)4 ] n(2),[Zn II 2 L 2(N 3)3 ] n(3)和[Cd II 2 L 1(N 3))3 ] n(4),其中HL 1和HL 2是席夫碱配体,是通过3-乙氧基水杨醛(对于HL 1)/ 3-甲氧基水杨醛(对于HL 2)和N,N,2 1:1缩合获得的,2-四甲基-1,3-丙二胺。化合物1是离散的Trizinc(II)系统,而其他三种化合物是一维配位聚合物,其建筑单元分别是Tricadmium(II),dizinc(II)和dicadmium(II)部分。HL 1的Zn II-叠氮基化合物和HL的Cd II-叠氮基化合物核的核原子核2是相似的(1和2是三核),类似地,所述锌II从HL -叠氮基化合物2和CD II从HL -叠氮化合物1是相似的(3和4是双核),揭示了两个不同寻常的结构相似在周期表中作为配位体周边略有变化的函数的两个不同的行的金属离子(OET在HL 1,OME在HL 2)。稳态/时间分辨的光物理性质表明,镉(II)略微增强,而锌(II)则显着增强席夫碱配体HL 1和HL的荧光2。已经发现HL 1和HL 2都是锌(II)的荧光传感器。































 京公网安备 11010802027423号
京公网安备 11010802027423号