Journal of Fluorine Chemistry ( IF 1.7 ) Pub Date : 2017-12-02 , DOI: 10.1016/j.jfluchem.2017.11.014 Helio G. Bonacorso , Melissa B. Rodrigues , Sarah C. Feitosa , Helena S. Coelho , Sydney H. Alves , Jéssica T. Keller , Wilian C. Rosa , Alex Ketzer , Clarissa P. Frizzo , Marcos A.P. Martins , Nilo Zanatta
|
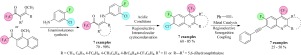
This paper firstly describes the synthesis of a series of (Z)-4-((3-chloro-4-fluorophenyl)amino)-1,1,1-trifluorobut-3-en-2-ones (3a–f) as well as 1-(1-((3-chloro-4-fluorophenyl)amino)-3,4-dihydronaphthalen-2-yl)-2,2,2-trifluoroethanone (3g), from the O,N-exchange reaction of some 4-methoxy-4-alkyl(aryl)-1,1,1-trifluoroalk-3-en-2-ones (1) with 3-chloro-4-fluoroaniline (2), at 70–90% yields. Subsequently, in concentrated sulfuric acid, the enaminoketones 3 underwent a successful and regioselective intramolecular cyclocondensation reaction, which furnished a novel series of 2-alkyl(aryl)-7-chloro-6-fluoro-4-(trifluoromethyl)-quinolines (4) at 70–85% yields. To demonstrate synthetic applicability through the Sonogashira coupling reaction for obtaining quinolines with potential antimicrobial activity, the synthesis of a novel series of 2-alkyl(aryl)-6-fluoro-7-(phenylethynyl)-4-(trifluoromethyl)-quinolines (5) was performed, using quinolines 4 and phenylacetylene, via a regioselective Sonogashira coupling reaction (25–50% yield). Unfortunately, the new series of heterocycles 4 and 5 did not produce significant results against bacteria and fungi at the concentrations of 80–0.31 μg/mL.
中文翻译:

Sonogashira交叉偶联反应促进的2-烷基(芳基)-7-氯-6-氟-4-(三氟甲基)-喹啉及其苯乙炔衍生物的合成和抗菌筛选
本文首先描述了一系列(Z)-4-((3-氯-4-氟苯基)氨基)-1,1,1-三氟丁-3-烯-2-酮(3a–f)的合成以及O,N交换反应中的1-(1-((3-氯-4-氟苯基)氨基)-3,4-二氢萘-2-基)-2,2,2-三氟乙酮(3g)用70-90%的产率将一些4-甲氧基-4-烷基(芳基)-1,1,1-三氟烷基-3-烯-2-酮(1)与3-氯-4-氟苯胺(2)结合使用。随后,在浓硫酸中,烯氨基酮3进行了成功的区域选择性分子内环缩合反应,从而提供了一系列新颖的2-烷基(芳基)-7-氯-6-氟-4-(三氟甲基)-喹啉(4)以70-85%的收益率。为了通过Sonogashira偶联反应证明具有潜在抗菌活性的喹啉的合成适用性,合成了一系列新型的2-烷基(芳基)-6-氟-7-(苯基乙炔基)-4-(三氟甲基)-喹啉(5)是通过喹啉4和苯乙炔通过区域选择性Sonogashira偶联反应(25-50%的收率)进行的。不幸的是,在浓度为80–0.31μg/ mL的情况下,新系列的杂环4和5并未对细菌和真菌产生显着结果。











































 京公网安备 11010802027423号
京公网安备 11010802027423号