Cell Systems ( IF 9.0 ) Pub Date : 2017-11-01 , DOI: 10.1016/j.cels.2017.10.004 Edward Y. Kim , Erin R. Tyndall , Kerwyn Casey Huang , Fang Tian , Kumaran S. Ramamurthi
|
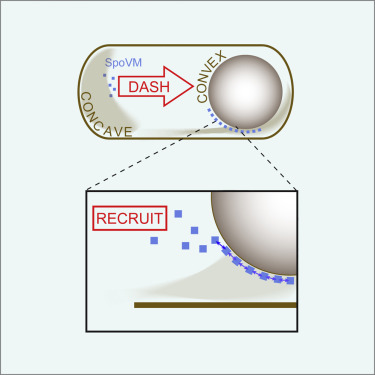
In Bacillus subtilis, sporulation requires that the 26-amino acid protein SpoVM embeds specifically into the forespore membrane, a structure with convex curvature. How this nanometer-sized protein can detect curves on a micrometer scale is not well understood. Here, we report that SpoVM exploits a “dash-and-recruit” mechanism to preferentially accumulate on the forespore. Using time-resolved imaging and flow cytometry, we observe that SpoVM exhibits a faster adsorption rate onto membranes of higher convex curvature. This preferential adsorption is accurately modeled as a two-step process: first, an initial binding event occurs with a faster on rate, then cooperative recruitment of additional SpoVM molecules follows. We demonstrate that both this biochemical process and effective sporulation in vivo require an unstructured and flexible SpoVM N terminus. We propose that this two-pronged strategy of fast adsorption followed by recruitment of subsequent molecules is a general mechanism that allows small proteins to detect subtle curves with a radius 1,000-fold their size.
中文翻译:

短跑和募集机制通过小细菌蛋白SpoVM驱动膜曲率识别。
在枯草芽孢杆菌中,孢子形成需要26个氨基酸的蛋白SpoVM专门嵌入到具有凸曲率的结构的前孢子膜中。这种纳米大小的蛋白质如何能够检测微米级的曲线还不是很了解。在这里,我们报告SpoVM利用“破折号”机制优先积累在前孢子上。使用时间分辨成像和流式细胞仪,我们观察到SpoVM在更高的凸曲率的膜上表现出更快的吸附速率。准确地将这种优先吸附建模为一个两步过程:首先,以更快的启动速率发生初始结合事件,然后随后协同募集其他SpoVM分子。我们证明了这种生化过程和有效的孢子形成体内需要非结构化且灵活的SpoVM N末端。我们建议这种快速吸收然后吸收后续分子的两管齐下的策略是一种通用机制,它允许小蛋白质检测半径为其大小的1000倍的细微曲线。































 京公网安备 11010802027423号
京公网安备 11010802027423号