当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2Reduction at Low Overpotential on Cu Electrodes Resulting from the Reduction of Thick Cu2O Films
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-04-20 , DOI: 10.1021/ja3010978 Christina W. Li 1 , Matthew W. Kanan 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-04-20 , DOI: 10.1021/ja3010978 Christina W. Li 1 , Matthew W. Kanan 1
Affiliation
|
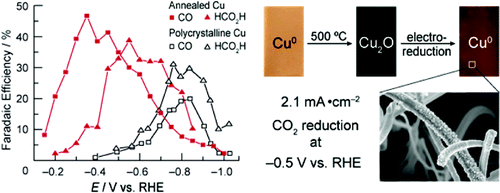
Modified Cu electrodes were prepared by annealing Cu foil in air and electrochemically reducing the resulting Cu(2)O layers. The CO(2) reduction activities of these electrodes exhibited a strong dependence on the initial thickness of the Cu(2)O layer. Thin Cu(2)O layers formed by annealing at 130 °C resulted in electrodes whose activities were indistinguishable from those of polycrystalline Cu. In contrast, Cu(2)O layers formed at 500 °C that were ≥~3 μm thick resulted in electrodes that exhibited large roughness factors and required 0.5 V less overpotential than polycrystalline Cu to reduce CO(2) at a higher rate than H(2)O. The combination of these features resulted in CO(2) reduction geometric current densities >1 mA/cm(2) at overpotentials <0.4 V, a higher level of activity than all previously reported metal electrodes evaluated under comparable conditions. Moreover, the activity of the modified electrodes was stable over the course of several hours, whereas a polycrystalline Cu electrode exhibited deactivation within 1 h under identical conditions. The electrodes described here may be particularly useful for elucidating the structural properties of Cu that determine the distribution between CO(2) and H(2)O reduction and provide a promising lead for the development of practical catalysts for electrolytic fuel synthesis.
中文翻译:

由厚的 Cu2O 薄膜的还原引起的 Cu 电极上低过电位的 CO2 还原
通过在空气中对铜箔进行退火和电化学还原所得的 Cu(2)O 层来制备改性 Cu 电极。这些电极的 CO(2) 还原活动表现出对 Cu(2)O 层初始厚度的强烈依赖。通过在 130 °C 下退火形成的薄 Cu(2)O 层导致电极的活性与多晶 Cu 的活性无法区分。相比之下,在 500 °C 下形成的 Cu(2)O 层厚度≥~3 μm 导致电极表现出较大的粗糙度系数,并且需要比多晶 Cu 低 0.5 V 的过电位,以比 H 更高的速率减少 CO(2) (2)O。这些功能的组合导致 CO(2) 减少几何电流密度 >1 mA/cm(2) 在过电位 <0.4 V,比在可比条件下评估的所有先前报告的金属电极更高的活性水平。此外,修饰电极的活性在几个小时内保持稳定,而多晶铜电极在相同条件下在 1 小时内表现出失活。此处描述的电极对于阐明决定 CO(2) 和 H(2)O 还原之间分布的 Cu 的结构特性可能特别有用,并为开发用于电解燃料合成的实用催化剂提供了有希望的线索。
更新日期:2012-04-20
中文翻译:

由厚的 Cu2O 薄膜的还原引起的 Cu 电极上低过电位的 CO2 还原
通过在空气中对铜箔进行退火和电化学还原所得的 Cu(2)O 层来制备改性 Cu 电极。这些电极的 CO(2) 还原活动表现出对 Cu(2)O 层初始厚度的强烈依赖。通过在 130 °C 下退火形成的薄 Cu(2)O 层导致电极的活性与多晶 Cu 的活性无法区分。相比之下,在 500 °C 下形成的 Cu(2)O 层厚度≥~3 μm 导致电极表现出较大的粗糙度系数,并且需要比多晶 Cu 低 0.5 V 的过电位,以比 H 更高的速率减少 CO(2) (2)O。这些功能的组合导致 CO(2) 减少几何电流密度 >1 mA/cm(2) 在过电位 <0.4 V,比在可比条件下评估的所有先前报告的金属电极更高的活性水平。此外,修饰电极的活性在几个小时内保持稳定,而多晶铜电极在相同条件下在 1 小时内表现出失活。此处描述的电极对于阐明决定 CO(2) 和 H(2)O 还原之间分布的 Cu 的结构特性可能特别有用,并为开发用于电解燃料合成的实用催化剂提供了有希望的线索。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号