当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Carrier Protein Strategy Yields the Structure of Dalbavancin
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-03-01 , DOI: 10.1021/ja208755j Nicoleta J Economou 1 , Virginie Nahoum , Stephen D Weeks , Kimberly C Grasty , Isaac J Zentner , Tracy M Townsend , Mohammad W Bhuiya , Simon Cocklin , Patrick J Loll
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-03-01 , DOI: 10.1021/ja208755j Nicoleta J Economou 1 , Virginie Nahoum , Stephen D Weeks , Kimberly C Grasty , Isaac J Zentner , Tracy M Townsend , Mohammad W Bhuiya , Simon Cocklin , Patrick J Loll
Affiliation
|
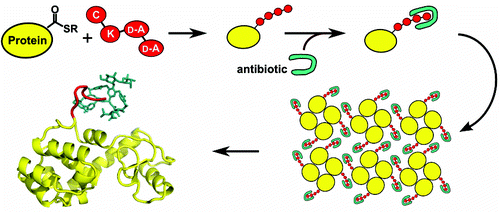
Many large natural product antibiotics act by specifically binding and sequestering target molecules found on bacterial cells. We have developed a new strategy to expedite the structural analysis of such antibiotic-target complexes, in which we covalently link the target molecules to carrier proteins, and then crystallize the entire carrier-target-antibiotic complex. Using native chemical ligation, we have linked the Lys-D-Ala-D-Ala binding epitope for glycopeptide antibiotics to three different carrier proteins. We show that recognition of this peptide by multiple antibiotics is not compromised by the presence of the carrier protein partner, and use this approach to determine the first-ever crystal structure for the new therapeutic dalbavancin. We also report the first crystal structure of an asymmetric ristocetin antibiotic dimer, as well as the structure of vancomycin bound to a carrier-target fusion. The dalbavancin structure reveals an antibiotic molecule that has closed around its binding partner; it also suggests mechanisms by which the drug can enhance its half-life by binding to serum proteins, and be targeted to bacterial membranes. Notably, the carrier protein approach is not limited to peptide ligands such as Lys-D-Ala-D-Ala, but is applicable to a diverse range of targets. This strategy is likely to yield structural insights that accelerate new therapeutic development.
中文翻译:

载体蛋白策略产生达巴万星的结构
许多大型天然产物抗生素通过特异性结合和隔离细菌细胞上发现的目标分子来发挥作用。我们开发了一种新策略来加快此类抗生素-靶标复合物的结构分析,其中我们将靶分子与载体蛋白共价连接,然后使整个载体-靶标-抗生素复合物结晶。使用天然化学连接,我们已将糖肽抗生素的 Lys-D-Ala-D-Ala 结合表位与三种不同的载体蛋白连接起来。我们表明,多种抗生素对这种肽的识别不会因载体蛋白伴侣的存在而受到影响,并使用这种方法来确定新治疗药物达巴万星的第一个晶体结构。我们还报告了不对称瑞斯托霉素抗生素二聚体的第一个晶体结构,以及与载体-靶标融合体结合的万古霉素的结构。达巴万星结构揭示了一种抗生素分子,它已在其结合伴侣周围闭合;它还提出了药物通过与血清蛋白结合并靶向细菌膜来延长其半衰期的机制。值得注意的是,载体蛋白方法不限于肽配体,如 Lys-D-Ala-D-Ala,而是适用于各种目标。这种策略可能会产生加速新疗法开发的结构性见解。值得注意的是,载体蛋白方法不限于肽配体,如 Lys-D-Ala-D-Ala,而是适用于各种目标。这种策略可能会产生加速新疗法开发的结构性见解。值得注意的是,载体蛋白方法不限于肽配体,如 Lys-D-Ala-D-Ala,而是适用于各种目标。这种策略可能会产生加速新疗法开发的结构性见解。
更新日期:2012-03-01
中文翻译:

载体蛋白策略产生达巴万星的结构
许多大型天然产物抗生素通过特异性结合和隔离细菌细胞上发现的目标分子来发挥作用。我们开发了一种新策略来加快此类抗生素-靶标复合物的结构分析,其中我们将靶分子与载体蛋白共价连接,然后使整个载体-靶标-抗生素复合物结晶。使用天然化学连接,我们已将糖肽抗生素的 Lys-D-Ala-D-Ala 结合表位与三种不同的载体蛋白连接起来。我们表明,多种抗生素对这种肽的识别不会因载体蛋白伴侣的存在而受到影响,并使用这种方法来确定新治疗药物达巴万星的第一个晶体结构。我们还报告了不对称瑞斯托霉素抗生素二聚体的第一个晶体结构,以及与载体-靶标融合体结合的万古霉素的结构。达巴万星结构揭示了一种抗生素分子,它已在其结合伴侣周围闭合;它还提出了药物通过与血清蛋白结合并靶向细菌膜来延长其半衰期的机制。值得注意的是,载体蛋白方法不限于肽配体,如 Lys-D-Ala-D-Ala,而是适用于各种目标。这种策略可能会产生加速新疗法开发的结构性见解。值得注意的是,载体蛋白方法不限于肽配体,如 Lys-D-Ala-D-Ala,而是适用于各种目标。这种策略可能会产生加速新疗法开发的结构性见解。值得注意的是,载体蛋白方法不限于肽配体,如 Lys-D-Ala-D-Ala,而是适用于各种目标。这种策略可能会产生加速新疗法开发的结构性见解。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号