当前位置:
X-MOL 学术
›
Org. Process Res. Dev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
New and Practical Synthesis of Gedatolisib
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.oprd.7b00298 Xiangyu Liu 1 , Guoqing Zhu 1 , Lingai Li 1 , Yaowei Liu 1 , Feng Wang 1 , Xiaoping Song 2 , Yongjun Mao 1
Organic Process Research & Development ( IF 3.1 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.oprd.7b00298 Xiangyu Liu 1 , Guoqing Zhu 1 , Lingai Li 1 , Yaowei Liu 1 , Feng Wang 1 , Xiaoping Song 2 , Yongjun Mao 1
Affiliation
|
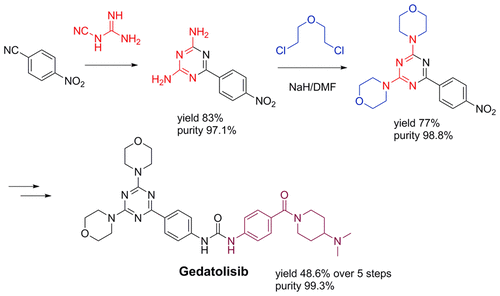
A new, practical, and convergent synthetic route of gedatolisib, an antitumor agent, is developed on a hectogram scale which avoids the Pd coupling method. The key step is adopting 6-(4-nitrophenyl)-1,3,5-triazine-2,4-diamine and 2,2′-dichlorodiethyl ether to prepare the key 4,4′-(6-(4-nitrophenyl)-1,3,5-triazine-2,4-diyl)dimorpholine in 77% yield and 98.8% purity. Gedatolisib is obtained in 48.6% yield over five simple steps and 99.3% purity (HPLC). Purification methods of the intermediates and the final product involved in the route are given.
中文翻译:

格达列西的新的实用合成
以百克级规模开发了一种新的,实用且聚合的抗肿瘤药gedatolisib合成路线,该路线避免了Pd偶联方法。关键步骤是采用6-(4-硝基苯基)-1,3,5-三嗪-2,4-二胺和2,2'-二氯二乙醚制备关键的4,4'-(6-(4-硝基苯基) )-1,3,5-三嗪-2,4-二基)二吗啉的产率为77%,纯度为98.8%。在五个简单的步骤中以98.6%的纯度(HPLC)获得了Gedatolisib的收率为48.6%。给出了该路线涉及的中间体和最终产物的纯化方法。
更新日期:2017-12-11
中文翻译:

格达列西的新的实用合成
以百克级规模开发了一种新的,实用且聚合的抗肿瘤药gedatolisib合成路线,该路线避免了Pd偶联方法。关键步骤是采用6-(4-硝基苯基)-1,3,5-三嗪-2,4-二胺和2,2'-二氯二乙醚制备关键的4,4'-(6-(4-硝基苯基) )-1,3,5-三嗪-2,4-二基)二吗啉的产率为77%,纯度为98.8%。在五个简单的步骤中以98.6%的纯度(HPLC)获得了Gedatolisib的收率为48.6%。给出了该路线涉及的中间体和最终产物的纯化方法。







































 京公网安备 11010802027423号
京公网安备 11010802027423号