当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Intramolecular Disulfide Bond between Catalytic Cysteines in an Intein Precursor
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-27 , DOI: 10.1021/ja211010g
Wen Chen 1 , Lingyun Li , Zhenming Du , Jiajing Liu , Julie N Reitter , Kenneth V Mills , Robert J Linhardt , Chunyu Wang
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-27 , DOI: 10.1021/ja211010g
Wen Chen 1 , Lingyun Li , Zhenming Du , Jiajing Liu , Julie N Reitter , Kenneth V Mills , Robert J Linhardt , Chunyu Wang
Affiliation
|
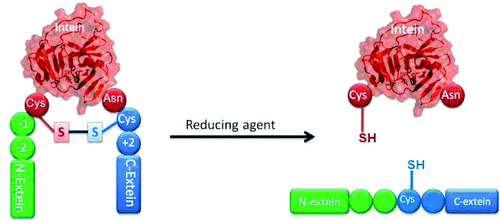
Protein splicing is a self-catalyzed and spontaneous post-translational process in which inteins excise themselves out of precursor proteins while the exteins are ligated together. We report the first discovery of an intramolecular disulfide bond between the two active-site cysteines, Cys1 and Cys+1, in an intein precursor composed of the hyperthermophilic Pyrococcus abyssi PolII intein and extein. The existence of this intramolecular disulfide bond is demonstrated by the effect of reducing agents on the precursor, mutagenesis, and liquid chromatography-mass spectrometry (LC-MS) with tandem MS (MS/MS) of the tryptic peptide containing the intramolecular disulfide bond. The disulfide bond inhibits protein splicing, and splicing can be induced by reducing agents such as tris(2-carboxyethyl)phosphine (TCEP). The stability of the intramolecular disulfide bond is enhanced by electrostatic interactions between the N- and C-exteins but is reduced by elevated temperature. The presence of this intramolecular disulfide bond may contribute to the redox control of splicing activity in hypoxia and at low temperature and point to the intriguing possibility that inteins may act as switches to control extein function.
中文翻译:

内含肽前体中催化半胱氨酸之间的分子内二硫键
蛋白质剪接是一种自催化和自发的翻译后过程,其中内含肽将自身从前体蛋白质中切除,同时外显肽连接在一起。我们报告了在由超嗜热 Pyrococcus abyssi PolII intein 和 extein 组成的内含肽前体中的两个活性位点半胱氨酸、Cys1 和 Cys+1 之间的分子内二硫键的首次发现。这种分子内二硫键的存在通过还原剂对含有分子内二硫键的胰蛋白酶肽的前体、诱变和液相色谱-质谱 (LC-MS) 与串联 MS (MS/MS) 的影响来证明。二硫键抑制蛋白质剪接,剪接可以通过还原剂如三(2-羧乙基)膦(TCEP)诱导。分子内二硫键的稳定性通过 N-和 C-exteins 之间的静电相互作用增强,但在升高的温度下会降低。这种分子内二硫键的存在可能有助于在缺氧和低温下对剪接活性的氧化还原控制,并指出内含肽可以作为控制外显肽功能的开关的有趣可能性。
更新日期:2012-01-27
中文翻译:

内含肽前体中催化半胱氨酸之间的分子内二硫键
蛋白质剪接是一种自催化和自发的翻译后过程,其中内含肽将自身从前体蛋白质中切除,同时外显肽连接在一起。我们报告了在由超嗜热 Pyrococcus abyssi PolII intein 和 extein 组成的内含肽前体中的两个活性位点半胱氨酸、Cys1 和 Cys+1 之间的分子内二硫键的首次发现。这种分子内二硫键的存在通过还原剂对含有分子内二硫键的胰蛋白酶肽的前体、诱变和液相色谱-质谱 (LC-MS) 与串联 MS (MS/MS) 的影响来证明。二硫键抑制蛋白质剪接,剪接可以通过还原剂如三(2-羧乙基)膦(TCEP)诱导。分子内二硫键的稳定性通过 N-和 C-exteins 之间的静电相互作用增强,但在升高的温度下会降低。这种分子内二硫键的存在可能有助于在缺氧和低温下对剪接活性的氧化还原控制,并指出内含肽可以作为控制外显肽功能的开关的有趣可能性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号