当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insight into the Common Mechanism of the Chromophore Formation in the Red Fluorescent Proteins: The Elusive Blue Intermediate Revealed
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-26 , DOI: 10.1021/ja2114568 Ksenia B Bravaya 1 , Oksana M Subach , Nadezhda Korovina , Vladislav V Verkhusha , Anna I Krylov
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2012-01-26 , DOI: 10.1021/ja2114568 Ksenia B Bravaya 1 , Oksana M Subach , Nadezhda Korovina , Vladislav V Verkhusha , Anna I Krylov
Affiliation
|
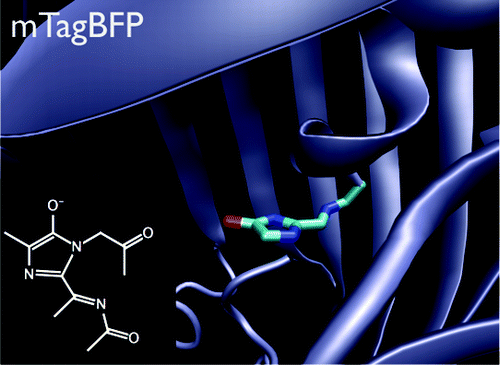
Understanding the chromophore maturation process in fluorescent proteins is important for the design of proteins with improved properties. Here, we present the results of electronic structure calculations identifying the nature of a blue intermediate, a key species in the process of the red chromophore formation in DsRed, TagRFP, fluorescent timers, and PAmCherry. The chromophore of the blue intermediate has a structure in which the π-system of the imidazole ring is extended by the acylimine bond, which can be represented by the model N-[(5-hydroxy-1H-imidazole-2yl)methylidene]acetamide (HIMA) compound. Ab initio and QM/MM calculations of the isolated model and protein-bound (mTagBFP) chromophores identify the anionic form of HIMA as the only structure that has absorption that is consistent with the experiment and is stable in the protein binding pocket. The anion and zwitterion are the only protonation forms of HIMA whose absorption (421 and 414 nm, or 2.95 and 3.00 eV) matches the experimental spectrum of the blue form in DsRed (the absorption maximum is 408 nm or 3.04 eV) and mTagBFP (400 nm or 3.10 eV). The QM/MM optimization of the protein-bound anionic form results in a structure that is close to the X-ray one, whereas the zwitterionic chromophore is unstable in the protein binding pocket and undergoes prompt proton transfer. The computed excitation energy of the protein-bound anionic form of the mTagBFP-like chromophore (3.04 eV) agrees with the experimental absorption spectrum of the protein. The DsRed-like chromophore formation in red fluorescent proteins is revisited on the basis of ab initio results and verified by directed mutagenesis revealing a key role of the amino acid residue 70, which is the second after the chromophore tripeptide, in the formation process.
中文翻译:

深入了解红色荧光蛋白中发色团形成的共同机制:揭示难以捉摸的蓝色中间体
了解荧光蛋白中发色团的成熟过程对于设计具有改进特性的蛋白质非常重要。在这里,我们展示了电子结构计算结果,确定了蓝色中间体的性质,蓝色中间体是 DsRed、TagRFP、荧光计时器和 PAmCherry 中红色发色团形成过程中的关键物质。蓝色中间体的发色团具有咪唑环的π-体系通过酰亚胺键延伸的结构,可以用模型N-[(5-羟基-1H-咪唑-2基)亚甲基]乙酰胺表示(HIMA)化合物。分离模型和蛋白质结合 (mTagBFP) 发色团的从头计算和 QM/MM 计算将 HIMA 的阴离子形式确定为唯一具有与实验一致的吸收且在蛋白质结合袋中稳定的结构。阴离子和两性离子是 HIMA 的唯一质子化形式,其吸收(421 和 414 nm,或 2.95 和 3.00 eV)与 DsRed 中蓝色形式的实验光谱(最大吸收为 408 nm 或 3.04 eV)和 mTagBFP(400 nm 或 3.10 eV)。蛋白质结合阴离子形式的 QM/MM 优化产生了接近 X 射线结构的结构,而两性离子发色团在蛋白质结合袋中不稳定并经历快速质子转移。计算出的 mTagBFP 类发色团的蛋白质结合阴离子形式的激发能 (3.04 eV) 与蛋白质的实验吸收光谱一致。 基于从头算结果重新审视红色荧光蛋白中 DsRed 样发色团的形成,并通过定向诱变进行验证,揭示了氨基酸残基 70(继发色团三肽之后的第二个)在形成过程中的关键作用。
更新日期:2012-01-26
中文翻译:

深入了解红色荧光蛋白中发色团形成的共同机制:揭示难以捉摸的蓝色中间体
了解荧光蛋白中发色团的成熟过程对于设计具有改进特性的蛋白质非常重要。在这里,我们展示了电子结构计算结果,确定了蓝色中间体的性质,蓝色中间体是 DsRed、TagRFP、荧光计时器和 PAmCherry 中红色发色团形成过程中的关键物质。蓝色中间体的发色团具有咪唑环的π-体系通过酰亚胺键延伸的结构,可以用模型N-[(5-羟基-1H-咪唑-2基)亚甲基]乙酰胺表示(HIMA)化合物。分离模型和蛋白质结合 (mTagBFP) 发色团的从头计算和 QM/MM 计算将 HIMA 的阴离子形式确定为唯一具有与实验一致的吸收且在蛋白质结合袋中稳定的结构。阴离子和两性离子是 HIMA 的唯一质子化形式,其吸收(421 和 414 nm,或 2.95 和 3.00 eV)与 DsRed 中蓝色形式的实验光谱(最大吸收为 408 nm 或 3.04 eV)和 mTagBFP(400 nm 或 3.10 eV)。蛋白质结合阴离子形式的 QM/MM 优化产生了接近 X 射线结构的结构,而两性离子发色团在蛋白质结合袋中不稳定并经历快速质子转移。计算出的 mTagBFP 类发色团的蛋白质结合阴离子形式的激发能 (3.04 eV) 与蛋白质的实验吸收光谱一致。 基于从头算结果重新审视红色荧光蛋白中 DsRed 样发色团的形成,并通过定向诱变进行验证,揭示了氨基酸残基 70(继发色团三肽之后的第二个)在形成过程中的关键作用。





























 京公网安备 11010802027423号
京公网安备 11010802027423号