当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemo- and Regioselectivity-Tunable Pd-Catalyzed Allylic Alkylation of Imines
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-09-14 , DOI: 10.1021/ja2039503 Jian-Ping Chen , Qian Peng , Bai-Lin Lei , Xue-Long Hou , Yun-Dong Wu 1, 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-09-14 , DOI: 10.1021/ja2039503 Jian-Ping Chen , Qian Peng , Bai-Lin Lei , Xue-Long Hou , Yun-Dong Wu 1, 2
Affiliation
|
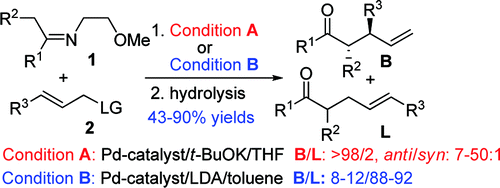
α-Carbanions of cyclic and acyclic imines have been successfully applied as nucleophiles in the Pd-catalyzed allylic alkylation reaction. Tuning of chemo- and regioselectivity has been realized by using t-BuOK/THF and LDA/toluene to give branched and linear products, respectively, with high regio- and diastereoselectivities. A plausible mechanism is proposed on the basis of the experimental results and DFT calculations.
中文翻译:

亚胺的化学和区域选择性可调的 Pd 催化烯丙基烷基化
环状和非环状亚胺的 α-碳负离子已成功用作 Pd 催化的烯丙基烷基化反应中的亲核试剂。通过使用 t-BuOK/THF 和 LDA/甲苯分别得到具有高区域选择性和非对映选择性的支化和线性产物,已经实现了化学和区域选择性的调节。根据实验结果和 DFT 计算提出了一种合理的机制。
更新日期:2011-09-14
中文翻译:

亚胺的化学和区域选择性可调的 Pd 催化烯丙基烷基化
环状和非环状亚胺的 α-碳负离子已成功用作 Pd 催化的烯丙基烷基化反应中的亲核试剂。通过使用 t-BuOK/THF 和 LDA/甲苯分别得到具有高区域选择性和非对映选择性的支化和线性产物,已经实现了化学和区域选择性的调节。根据实验结果和 DFT 计算提出了一种合理的机制。






























 京公网安备 11010802027423号
京公网安备 11010802027423号