当前位置:
X-MOL 学术
›
Angew. Chem. Int. Ed.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
General and Facile Route to Isomerically Pure Tricyclic Peptides Based on Templated Tandem CLIPS/CuAAC Cyclizations
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-12-14 , DOI: 10.1002/anie.201709127 Gaston J. J. Richelle 1 , Sumeet Ori 1 , Henk Hiemstra 1 , Jan H. van Maarseveen 1 , Peter Timmerman 2
Angewandte Chemie International Edition ( IF 16.1 ) Pub Date : 2017-12-14 , DOI: 10.1002/anie.201709127 Gaston J. J. Richelle 1 , Sumeet Ori 1 , Henk Hiemstra 1 , Jan H. van Maarseveen 1 , Peter Timmerman 2
Affiliation
|
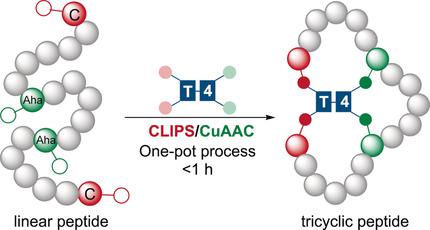
We report a one‐pot ligation/cyclization technology for the rapid and clean conversion of linear peptides into tricyclic peptides that is based on using tetravalent scaffolds containing two benzyl bromide and two alkyne moieties. These react via CLIPS/CuAAC reactions with cysteines and azides in the peptide. Flexibility in the scaffolds is key to the formation of isomerically pure products as the flexible scaffolds T41 and T42 mostly promote the formation of single isomeric tricycles while the rigid scaffolds T43 and T44 do not yield clean products. There seems to be no limitation to the number and types of amino acids present as 18 canonical amino acids were successfully implemented. We also observed that azides at the peptide termini and cysteine residues in the center gave better results than compounds with the functional groups placed the other way round.
中文翻译:

基于模板化串联CLIPS / CuAAC环化的异构纯三环肽的通用和简便途径。
我们报告了一种基于线性的肽快速,干净地转化为三环肽的单罐连接/环化技术,该技术基于使用包含两个苄基溴和两个炔基的四价支架。它们通过CLIPS / CuAAC反应与肽中的半胱氨酸和叠氮化物反应。支架的柔韧性是形成异构异构纯产物的关键,因为柔性支架T4 1和T4 2主要促进单个异构三轮车的形成,而刚性支架T4 3和T4 4不生产清洁产品。由于成功实现了18种规范氨基酸,似乎对氨基酸的数量和类型没有限制。我们还观察到,与末端带有官能团的化合物相比,位于肽末端和半胱氨酸中心的叠氮化物提供了更好的结果。
更新日期:2017-12-14
中文翻译:

基于模板化串联CLIPS / CuAAC环化的异构纯三环肽的通用和简便途径。
我们报告了一种基于线性的肽快速,干净地转化为三环肽的单罐连接/环化技术,该技术基于使用包含两个苄基溴和两个炔基的四价支架。它们通过CLIPS / CuAAC反应与肽中的半胱氨酸和叠氮化物反应。支架的柔韧性是形成异构异构纯产物的关键,因为柔性支架T4 1和T4 2主要促进单个异构三轮车的形成,而刚性支架T4 3和T4 4不生产清洁产品。由于成功实现了18种规范氨基酸,似乎对氨基酸的数量和类型没有限制。我们还观察到,与末端带有官能团的化合物相比,位于肽末端和半胱氨酸中心的叠氮化物提供了更好的结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号