当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Thermochemistry of the Initial Steps of Methylaluminoxane Formation. Aluminoxanes and Cycloaluminoxanes by Methane Elimination from Dimethylaluminum Hydroxide and Its Dimeric Aggregates
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-08-31 , DOI: 10.1021/ja109457j Rainer Glaser 1 , Xinsen Sun 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-08-31 , DOI: 10.1021/ja109457j Rainer Glaser 1 , Xinsen Sun 1
Affiliation
|
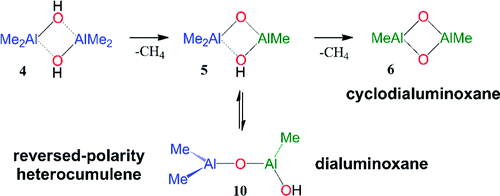
Results are presented of ab initio studies at levels MP2(full)/6-31G* and MP2(full)/6-311G** of the hydrolysis of trimethylaluminum (TMA, 1) to dimethylaluminumhydroxide (DMAH, 2) and of the intramolecular 1,2-elimination of CH(4) from 2 itself to form methylaluminumoxide 3, from its dimeric aggregate 4 to form hydroxytrimethyldialuminoxane 5 and dimethylcyclodialuminoxane 6, and from its TMA aggregate 7 to form 8 and/or 9, the cyclic and open isomers of tetramethyldialuminoxane, respectively. Each methane elimination creates one new Lewis acid site, and dimethylether is used as a model oxygen-donor molecule to assess the most important effects of product stabilization by Lewis donor coordination. It is found that the irreversible formation of aggregate 4 (ΔG(298) = -29.2 kcal/mol) is about three times more exergonic than the reversible formation of aggregate 7 (ΔG(298) = -9.9 kcal/mol), that the reaction free enthalpies for the formations of 5 (ΔG(298) = -9.0 kcal/mol) and 6 (ΔG(298) = -18.8 kcal/mol) both are predicted to be quite clearly exergonic, and that there is a significant thermodynamic preference (ΔG(298) = -7.2 kcal/mol) for the formation of 6 over ring-opening of 5 to hydroxytrimethyldialuminoxane 10. The mechanism for oligomerization is discussed based on the bonding properties of dimeric aggregates and involves the homologation of HO-free aluminoxane with DMAH (i.e., 9 to 13), and any initially formed hydroxydialuminoxane 10 is easily capped to trialuminoxane 13. Our studies are consistent with and provide support for Sinn's proposal for the formation of oligoaluminoxanes, and in addition, the results point to the crucial role played by the kinetic stability of 5 and the possibility to form cyclodialuminoxane 6. Dialuminoxanes 9 and 10 are reversed-polarity heterocumulenes, and intramolecular O→Al dative bonding competes successfully with Al complexation by Lewis donors. Intramolecular O→Al dative bonding is impeded in cyclodialuminoxane 6, and the dicoordinate oxygen in 6 is a strong Lewis donor. Ethylene polymerization catalysts contain highly oxophilic transition metals, and our studies suggest that these transition metal catalysts should discriminate strongly in favor of cycloaluminoxane-O donors even if these are present only in small concentrations in the methylaluminoxane (MAO) cocatalyst.
中文翻译:

甲基铝氧烷形成初始步骤的热化学。从二甲基氢氧化铝及其二聚体中去除甲烷的铝氧烷和环铝氧烷
结果显示在 MP2(full)/6-31G* 和 MP2(full)/6-311G** 水平下三甲基铝 (TMA, 1) 水解为二甲基氢氧化铝 (DMAH, 2) 和分子内1,2-从 2 本身消除 CH(4) 形成甲基铝氧化物 3,从其二聚体聚集体 4 消除形成羟基三甲基二铝氧烷 5 和二甲基环二铝氧烷 6,从其 TMA 聚集体 7 消除形成 8 和/或 9,环状和开环异构体分别为四甲基二铝氧烷。每次甲烷消除都会产生一个新的路易斯酸位点,二甲醚被用作模型氧供体分子,以评估路易斯供体配位对产品稳定性的最重要影响。发现不可逆形成聚集体4(ΔG(298) = -29。结果表明 5 的动力学稳定性和形成环二铝氧烷 6 的可能性起着至关重要的作用。二铝氧烷 9 和 10 是反极性的杂枯烯,分子内 O→Al 配价键合成功地与路易斯供体的铝络合竞争。分子内O→Al配键在环二铝氧烷6中受阻,6中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。二铝氧烷 9 和 10 是反极性杂枯烯,分子内 O→Al 配键与路易斯供体的 Al 络合成功竞争。环二铝氧烷 6 中的分子内 O→Al 配价键合受阻,6 中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。二铝氧烷 9 和 10 是反极性杂枯烯,分子内 O→Al 配键与路易斯供体的 Al 络合成功竞争。环二铝氧烷 6 中的分子内 O→Al 配价键合受阻,6 中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以很小的浓度存在。6中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。6中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。
更新日期:2011-08-31
中文翻译:

甲基铝氧烷形成初始步骤的热化学。从二甲基氢氧化铝及其二聚体中去除甲烷的铝氧烷和环铝氧烷
结果显示在 MP2(full)/6-31G* 和 MP2(full)/6-311G** 水平下三甲基铝 (TMA, 1) 水解为二甲基氢氧化铝 (DMAH, 2) 和分子内1,2-从 2 本身消除 CH(4) 形成甲基铝氧化物 3,从其二聚体聚集体 4 消除形成羟基三甲基二铝氧烷 5 和二甲基环二铝氧烷 6,从其 TMA 聚集体 7 消除形成 8 和/或 9,环状和开环异构体分别为四甲基二铝氧烷。每次甲烷消除都会产生一个新的路易斯酸位点,二甲醚被用作模型氧供体分子,以评估路易斯供体配位对产品稳定性的最重要影响。发现不可逆形成聚集体4(ΔG(298) = -29。结果表明 5 的动力学稳定性和形成环二铝氧烷 6 的可能性起着至关重要的作用。二铝氧烷 9 和 10 是反极性的杂枯烯,分子内 O→Al 配价键合成功地与路易斯供体的铝络合竞争。分子内O→Al配键在环二铝氧烷6中受阻,6中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。二铝氧烷 9 和 10 是反极性杂枯烯,分子内 O→Al 配键与路易斯供体的 Al 络合成功竞争。环二铝氧烷 6 中的分子内 O→Al 配价键合受阻,6 中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。二铝氧烷 9 和 10 是反极性杂枯烯,分子内 O→Al 配键与路易斯供体的 Al 络合成功竞争。环二铝氧烷 6 中的分子内 O→Al 配价键合受阻,6 中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以很小的浓度存在。6中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。6中的双配位氧是强路易斯供体。乙烯聚合催化剂含有高度亲氧的过渡金属,我们的研究表明,这些过渡金属催化剂应该强烈区分环铝氧烷-O 供体,即使它们在甲基铝氧烷 (MAO) 助催化剂中仅以小浓度存在。































 京公网安备 11010802027423号
京公网安备 11010802027423号