当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Mechanism of Water Oxidation with Single-Site Ruthenium–Heteropolytungstate Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-08-03 , DOI: 10.1021/ja2024965 Masato Murakami 1 , Dachao Hong 1 , Tomoyoshi Suenobu 1 , Satoru Yamaguchi 2 , Takashi Ogura 2 , Shunichi Fukuzumi 1, 3
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-08-03 , DOI: 10.1021/ja2024965 Masato Murakami 1 , Dachao Hong 1 , Tomoyoshi Suenobu 1 , Satoru Yamaguchi 2 , Takashi Ogura 2 , Shunichi Fukuzumi 1, 3
Affiliation
|
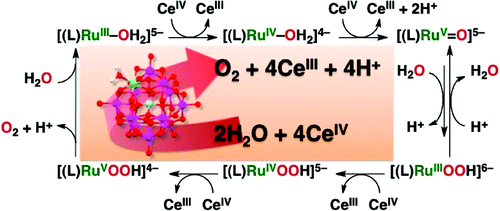
Catalytic water oxidation to generate oxygen was achieved using all-inorganic mononuclear ruthenium complexes bearing Keggin-type lacunary heteropolytungstate, [Ru(III)(H(2)O)SiW(11)O(39)](5-) (1) and [Ru(III)(H(2)O)GeW(11)O(39)](5-) (2), as catalysts with (NH(4))(2)[Ce(IV)(NO(3))(6)] (CAN) as a one-electron oxidant in water. The oxygen atoms of evolved oxygen come from water as confirmed by isotope-labeled experiments. Cyclic voltammetric measurements of 1 and 2 at various pH's indicate that both complexes 1 and 2 exhibit three one-electron redox couples based on ruthenium center. The Pourbaix diagrams (plots of E(1/2) vs pH) support that the Ru(III) complexes are oxidized to the Ru(V)-oxo complexes with CAN. The Ru(V)-oxo complex derived from 1 was detected by UV-visible absorption, EPR, and resonance Raman measurements in situ as an active species during the water oxidation reaction. This indicates that the Ru(V)-oxo complex is involved in the rate-determining step of the catalytic cycle of water oxidation. The overall catalytic mechanism of water oxidation was revealed on the basis of the kinetic analysis and detection of the catalytic intermediates. Complex 2 exhibited a higher catalytic reactivity for the water oxidation with CAN than did complex 1.
中文翻译:

单中心钌-杂多钨酸盐配合物氧化水的催化机理
使用带有 Keggin 型缺位杂多钨酸盐 [Ru(III)(H(2)O)SiW(11)O(39)](5-) (1) 的全无机单核钌配合物实现催化水氧化以产生氧气和 [Ru(III)(H(2)O)GeW(11)O(39)](5-) (2),作为催化剂与 (NH(4))(2)[Ce(IV)(NO( 3))(6)] (CAN) 作为水中的单电子氧化剂。同位素标记实验证实,析出的氧的氧原子来自水。1 和 2 在不同 pH 值下的循环伏安测量表明,配合物 1 和 2 均表现出三个基于钌中心的单电子氧化还原对。普贝图(E(1/2) 与 pH 的关系图)支持 Ru(III) 配合物被 CAN 氧化为 Ru(V)-氧代配合物。由 1 衍生的 Ru(V)-oxo 络合物通过紫外可见吸收、EPR、和原位共振拉曼测量作为水氧化反应过程中的活性物质。这表明 Ru(V)-oxo 络合物参与了水氧化催化循环的速率决定步骤。通过对催化中间体的动力学分析和检测,揭示了水氧化的整体催化机理。与配合物 1 相比,配合物 2 对 CAN 的水氧化表现出更高的催化反应性。
更新日期:2011-08-03
中文翻译:

单中心钌-杂多钨酸盐配合物氧化水的催化机理
使用带有 Keggin 型缺位杂多钨酸盐 [Ru(III)(H(2)O)SiW(11)O(39)](5-) (1) 的全无机单核钌配合物实现催化水氧化以产生氧气和 [Ru(III)(H(2)O)GeW(11)O(39)](5-) (2),作为催化剂与 (NH(4))(2)[Ce(IV)(NO( 3))(6)] (CAN) 作为水中的单电子氧化剂。同位素标记实验证实,析出的氧的氧原子来自水。1 和 2 在不同 pH 值下的循环伏安测量表明,配合物 1 和 2 均表现出三个基于钌中心的单电子氧化还原对。普贝图(E(1/2) 与 pH 的关系图)支持 Ru(III) 配合物被 CAN 氧化为 Ru(V)-氧代配合物。由 1 衍生的 Ru(V)-oxo 络合物通过紫外可见吸收、EPR、和原位共振拉曼测量作为水氧化反应过程中的活性物质。这表明 Ru(V)-oxo 络合物参与了水氧化催化循环的速率决定步骤。通过对催化中间体的动力学分析和检测,揭示了水氧化的整体催化机理。与配合物 1 相比,配合物 2 对 CAN 的水氧化表现出更高的催化反应性。






























 京公网安备 11010802027423号
京公网安备 11010802027423号