当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cobalt- and Silver-Promoted Methylenecyclopropane Rearrangements
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.joc.7b02477
Xavier Creary 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-12-11 00:00:00 , DOI: 10.1021/acs.joc.7b02477
Xavier Creary 1
Affiliation
|
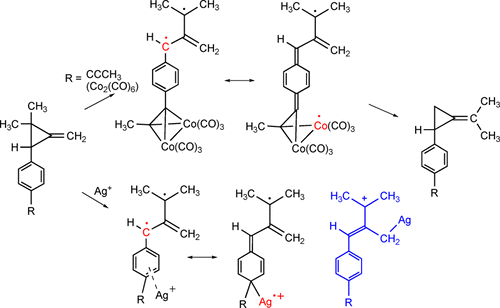
The rate of the methylenecyclopropane rearrangement is enhanced by an alkyne–Co2(CO)6 complex bonded to the para position of a benzene ring. This is explained by a stabilizing effect on the transition state leading to the biradical intermediate. Computational studies indicate that the benzylic-type biradical intermediate is stabilized by a delocalization mechanism, where spin is delocalized onto the two cobalt atoms. Silver cation also enhances the rate of the methylenecyclopropane rearrangement. Computational studies suggest that silver cation can also stabilize a benzylic radical by spin delocalization involving silver. In the case of the silver-promoted reactions, the rate enhancements in a series of aryl-substituted methylenecyclopropanes correlate with σ+ values. The question remains open as to whether the silver-catalyzed methylenecyclopropane rearrangement proceeds via an argento-stabilized biradical or whether the reaction involves an argento-substituted allylic cation.
中文翻译:

钴和银促进的亚甲基环丙烷重排
通过与苯环对位键合的炔烃-Co 2(CO)6络合物可提高亚甲基环丙烷重排的速率。这可以通过对导致双自由基中间体的过渡态的稳定作用来解释。计算研究表明,苄基型双自由基中间体是通过离域机制稳定的,其中自旋被离域到两个钴原子上。银阳离子还提高了亚甲基环丙烷重排的速率。计算研究表明,银阳离子还可以通过涉及银的自旋离域而稳定苄基。在银促进的反应中,一系列芳基取代的亚甲基环丙烷的速率提高与σ +相关。价值观。关于银催化的亚甲基环丙烷重排是否经由Argantto稳定的双自由基进行,或者该反应是否涉及Argantto取代的烯丙基阳离子,问题仍然悬而未决。
更新日期:2017-12-11
中文翻译:

钴和银促进的亚甲基环丙烷重排
通过与苯环对位键合的炔烃-Co 2(CO)6络合物可提高亚甲基环丙烷重排的速率。这可以通过对导致双自由基中间体的过渡态的稳定作用来解释。计算研究表明,苄基型双自由基中间体是通过离域机制稳定的,其中自旋被离域到两个钴原子上。银阳离子还提高了亚甲基环丙烷重排的速率。计算研究表明,银阳离子还可以通过涉及银的自旋离域而稳定苄基。在银促进的反应中,一系列芳基取代的亚甲基环丙烷的速率提高与σ +相关。价值观。关于银催化的亚甲基环丙烷重排是否经由Argantto稳定的双自由基进行,或者该反应是否涉及Argantto取代的烯丙基阳离子,问题仍然悬而未决。

































 京公网安备 11010802027423号
京公网安备 11010802027423号