当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct Observation of Photoinduced Ultrafast Generation of Singlet and Triplet Quinone Methides in Aqueous Solutions and Insight into the Roles of Acidic and Basic Sites in Quinone Methide Formation
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-11 , DOI: 10.1021/jacs.7b10387
Jiani Ma 1 , Xiting Zhang 2 , Nikola Basarić 3 , David Lee Phillips 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-11 , DOI: 10.1021/jacs.7b10387
Jiani Ma 1 , Xiting Zhang 2 , Nikola Basarić 3 , David Lee Phillips 2
Affiliation
|
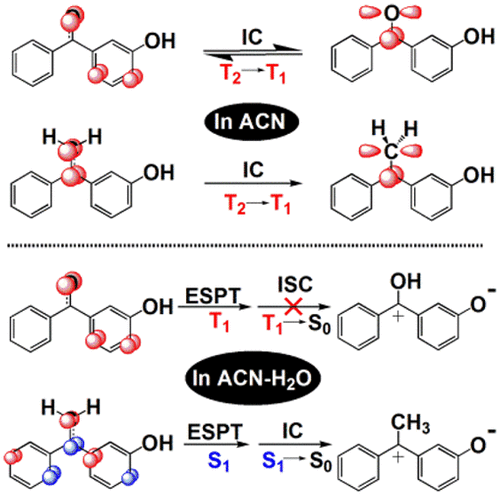
Femtosecond time-resolved transient absorption spectroscopy experiments and density functional theory computations were done for a mechanistic investigation of 3-(1-phenylvinyl)phenol (1) and 3-hydroxybenzophenone (2) in selected solvents. Both compounds went through an intersystem crossing (ISC) to form the triplet excited states Tππ* and Tnπ* in acetonitrile but behave differently in neutral aqueous solutions, in which a triplet excited state proton transfer (ESPT) induced by the ISC process is also proposed for 2 but a singlet ESPT without ISC is proposed for 1, leading to the production of the triplet quinone methide (QM) and the singlet excited QM species respectively in these two systems. The triplet QM then underwent an ISC process to form an unstable ground state intermediate which soon returned to its starting material 2. However, the singlet excited state QM went through an internal conversion process to the ground state QM followed by the formation of its final product in an irreversible manner. These differences are thought to be derived from the slow vinyl C-C rotation and the moderate basicity of the vinyl C atom in 1 as compared with the fast C-O rotation and the greater basicity of the carbonyl O atom of 2 after photoexcitation. This can account for the experimental results in the literature that the aromatic vinyl compounds undergo efficient singlet excited state photochemical reactions while the aromatic carbonyl compounds prefer triplet photochemical reactions under aqueous conditions. These results have fundamental and significant implications for understanding of the ESPT reactivity in general, as well as for the design of molecules for efficient QM formation in aqueous media with potential applications in cancer phototherapy.
中文翻译:

直接观察水溶液中单线态和三线态醌甲基化物的光诱导超快生成,并深入了解酸性和碱性位点在醌甲基化物形成中的作用
飞秒时间分辨瞬态吸收光谱实验和密度泛函理论计算用于对选定溶剂中的 3-(1-苯基乙烯基)苯酚 (1) 和 3-羟基二苯甲酮 (2) 进行机械研究。两种化合物都经过系统间交叉 (ISC) 在乙腈中形成三重激发态 Tππ* 和 Tnπ*,但在中性水溶液中表现不同,其中还提出了由 ISC 过程诱导的三重激发态质子转移 (ESPT)对于 2,但建议 1 没有 ISC 的单线态 ESPT,导致在这两个系统中分别产生三线态醌甲基化物 (QM) 和单线态激发 QM 物种。然后,三重态 QM 经历了 ISC 过程以形成不稳定的基态中间体,该中间体很快返回到其起始材料 2。然而,单重激发态 QM 经历了一个内部转换过程到基态 QM,然后以不可逆的方式形成其最终产物。这些差异被认为是由于与快速 CO 旋转和光激发后 2 的羰基 O 原子的更大碱性相比,1 中乙烯基 C 原子的慢速乙烯基 C 旋转和中等碱性。这可以解释文献中的实验结果,即芳族乙烯基化合物进行有效的单线激发态光化学反应,而芳族羰基化合物更喜欢在水性条件下进行三线态光化学反应。这些结果对于理解一般的 ESPT 反应性具有根本和重要的意义,
更新日期:2017-12-11
中文翻译:

直接观察水溶液中单线态和三线态醌甲基化物的光诱导超快生成,并深入了解酸性和碱性位点在醌甲基化物形成中的作用
飞秒时间分辨瞬态吸收光谱实验和密度泛函理论计算用于对选定溶剂中的 3-(1-苯基乙烯基)苯酚 (1) 和 3-羟基二苯甲酮 (2) 进行机械研究。两种化合物都经过系统间交叉 (ISC) 在乙腈中形成三重激发态 Tππ* 和 Tnπ*,但在中性水溶液中表现不同,其中还提出了由 ISC 过程诱导的三重激发态质子转移 (ESPT)对于 2,但建议 1 没有 ISC 的单线态 ESPT,导致在这两个系统中分别产生三线态醌甲基化物 (QM) 和单线态激发 QM 物种。然后,三重态 QM 经历了 ISC 过程以形成不稳定的基态中间体,该中间体很快返回到其起始材料 2。然而,单重激发态 QM 经历了一个内部转换过程到基态 QM,然后以不可逆的方式形成其最终产物。这些差异被认为是由于与快速 CO 旋转和光激发后 2 的羰基 O 原子的更大碱性相比,1 中乙烯基 C 原子的慢速乙烯基 C 旋转和中等碱性。这可以解释文献中的实验结果,即芳族乙烯基化合物进行有效的单线激发态光化学反应,而芳族羰基化合物更喜欢在水性条件下进行三线态光化学反应。这些结果对于理解一般的 ESPT 反应性具有根本和重要的意义,


































 京公网安备 11010802027423号
京公网安备 11010802027423号