当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boronic Acid Hydrogen Bonding in Encapsulation Complexes
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-06-29 , DOI: 10.1021/ja203123k
Dariush Ajami 1 , Henry Dube 1 , Julius Rebek 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-06-29 , DOI: 10.1021/ja203123k
Dariush Ajami 1 , Henry Dube 1 , Julius Rebek 1
Affiliation
|
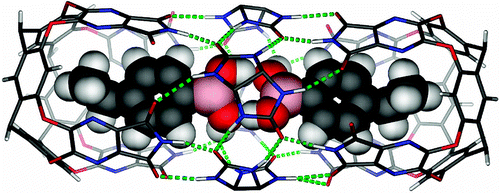
Hydrogen bonding is a key determinant of much macromolecular structure in nature, but individual donor and acceptor pairs are rarely observed in solution. Their weak interactions result in nanosecond lifetimes and rapid exchange of partners. Reversible encapsulation isolates molecules in very small spaces for milliseconds to hours and allows their characterization by NMR methods. Here we report a competitive study of hydrogen-bonding functions--carboxylic acids, primary amides, and boronic acids--within a multicomponent capsular assembly. The pairwise co-encapsulation of these molecules allows the direct observation of homodimeric boronic acids and their heterodimeric complexes with carboxylic acids and primary amides. The efficiency of boronic acids as hydrogen-bonding partners derives from their adaptable structures rather than from their intrinsic acid/base properties.
中文翻译:

包封复合物中的硼酸氢键
氢键是自然界中许多大分子结构的关键决定因素,但在溶液中很少观察到单独的供体和受体对。它们微弱的相互作用导致了纳秒级的寿命和伙伴的快速交换。可逆封装将分子隔离在非常小的空间内几毫秒到几小时,并允许通过 NMR 方法对其进行表征。在这里,我们报告了对多组分胶囊组件中氢键功能(羧酸、伯酰胺和硼酸)的竞争性研究。这些分子的成对共包封允许直接观察同二聚硼酸及其与羧酸和伯酰胺的异二聚复合物。
更新日期:2011-06-29
中文翻译:

包封复合物中的硼酸氢键
氢键是自然界中许多大分子结构的关键决定因素,但在溶液中很少观察到单独的供体和受体对。它们微弱的相互作用导致了纳秒级的寿命和伙伴的快速交换。可逆封装将分子隔离在非常小的空间内几毫秒到几小时,并允许通过 NMR 方法对其进行表征。在这里,我们报告了对多组分胶囊组件中氢键功能(羧酸、伯酰胺和硼酸)的竞争性研究。这些分子的成对共包封允许直接观察同二聚硼酸及其与羧酸和伯酰胺的异二聚复合物。

































 京公网安备 11010802027423号
京公网安备 11010802027423号