当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chiral Brønsted Acid-Catalyzed Asymmetric Trisubstituted Aziridine Synthesis Using α-Diazoacyl Oxazolidinones
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-06-29 , DOI: 10.1021/ja203901h Takuya Hashimoto 1 , Hiroki Nakatsu 1 , Kumiko Yamamoto 1 , Keiji Maruoka 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-06-29 , DOI: 10.1021/ja203901h Takuya Hashimoto 1 , Hiroki Nakatsu 1 , Kumiko Yamamoto 1 , Keiji Maruoka 1
Affiliation
|
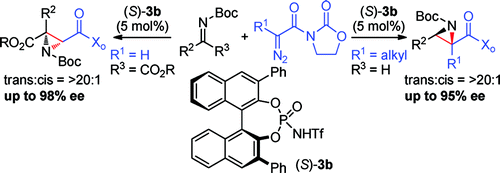
Despite the remarkable advances in catalytic asymmetric aziridinations over the past decades, establishing a general procedure for the stereoselective synthesis of trisubstituted aziridines has remained an elusive goal. Chiral N-triflyl phosphoramide-catalyzed reactions of N-α-diazoacyl oxazolidinones and N-Boc imines were developed as a solution to this unmet challenge.
中文翻译:

使用 α-重氮酰基恶唑烷酮合成手性布朗斯台德酸催化的不对称三取代氮丙啶
尽管在过去几十年中催化不对称氮丙啶化反应取得了显着进展,但建立立体选择性合成三取代氮丙啶的通用程序仍然是一个难以实现的目标。N-α-重氮酰基恶唑烷酮和 N-Boc 亚胺的手性 N-三氟甲基磷酰胺催化反应被开发为解决这一未满足挑战的解决方案。
更新日期:2011-06-29
中文翻译:

使用 α-重氮酰基恶唑烷酮合成手性布朗斯台德酸催化的不对称三取代氮丙啶
尽管在过去几十年中催化不对称氮丙啶化反应取得了显着进展,但建立立体选择性合成三取代氮丙啶的通用程序仍然是一个难以实现的目标。N-α-重氮酰基恶唑烷酮和 N-Boc 亚胺的手性 N-三氟甲基磷酰胺催化反应被开发为解决这一未满足挑战的解决方案。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号