当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Fragmentation–Recombination Mechanism of the Enzyme Glutamate Mutase Studied by QM/MM Simulations
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-07-06 , DOI: 10.1021/ja202312d Judith B. Rommel 1 , Johannes Kästner 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2011-07-06 , DOI: 10.1021/ja202312d Judith B. Rommel 1 , Johannes Kästner 1
Affiliation
|
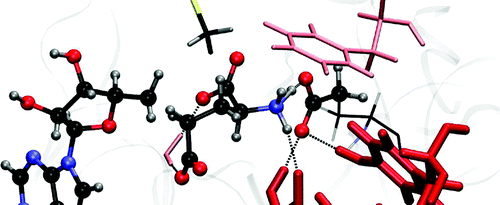
The radical mechanism of the conversion of glutamate to methylaspartate catalyzed by glutamate mutase is studied with quantum mechanical/molecular mechanical (QM/MM) simulations based on density functional theory (DFT/MM). The hydrogen transfer between the substrate and the cofactor is found to be rate limiting with a barrier of 101.1 kJ mol(-1). A careful comparison to the uncatalyzed reaction in water is performed. The protein influences the reaction predominantly electrostatically and to a lesser degree sterically. Our calculations shed light on the atomistic details of the reaction mechanism. The well-known arginine claw and Glu 171 ( Clostridium cochlearium notation) are found to have the strongest influence on the reaction. However, a catalytic role of Glu 214, Lys 322, Gln 147, Glu 330, Lys 326, and Met 294 is found as well. The arginine claw keeps the intermediates in place and is probably responsible for the enantioselectivity. Glu 171 temporarily accepts a proton from the glutamyl radical intermediate and donates it back at the end of the reaction. We relate our results to experimental data when available. Our simulations lead to further understanding of how glutamate mutase catalyzes the carbon skeleton rearrangement of glutamate.
中文翻译:

通过 QM/MM 模拟研究谷氨酸变位酶的断裂-重组机制
基于密度泛函理论 (DFT/MM) 的量子力学/分子力学 (QM/MM) 模拟研究了谷氨酸变位酶催化谷氨酸转化为甲基天冬氨酸的自由基机制。发现底物和辅因子之间的氢转移是限速的,屏障为 101.1 kJ mol(-1)。与在水中的未催化反应进行了仔细比较。蛋白质主要以静电方式影响反应,并在较小程度上影响空间。我们的计算揭示了反应机制的原子细节。发现众所周知的精氨酸爪和 Glu 171(耳蜗梭菌符号)对反应的影响最强。然而,也发现了 Glu 214、Lys 322、Gln 147、Glu 330、Lys 326 和 Met 294 的催化作用。精氨酸爪将中间体保持在原位,可能是对映选择性的原因。Glu 171 暂时接受来自谷氨酰自由基中间体的质子,并在反应结束时将其返回。我们将我们的结果与可用的实验数据联系起来。我们的模拟有助于进一步了解谷氨酸变位酶如何催化谷氨酸的碳骨架重排。
更新日期:2011-07-06
中文翻译:

通过 QM/MM 模拟研究谷氨酸变位酶的断裂-重组机制
基于密度泛函理论 (DFT/MM) 的量子力学/分子力学 (QM/MM) 模拟研究了谷氨酸变位酶催化谷氨酸转化为甲基天冬氨酸的自由基机制。发现底物和辅因子之间的氢转移是限速的,屏障为 101.1 kJ mol(-1)。与在水中的未催化反应进行了仔细比较。蛋白质主要以静电方式影响反应,并在较小程度上影响空间。我们的计算揭示了反应机制的原子细节。发现众所周知的精氨酸爪和 Glu 171(耳蜗梭菌符号)对反应的影响最强。然而,也发现了 Glu 214、Lys 322、Gln 147、Glu 330、Lys 326 和 Met 294 的催化作用。精氨酸爪将中间体保持在原位,可能是对映选择性的原因。Glu 171 暂时接受来自谷氨酰自由基中间体的质子,并在反应结束时将其返回。我们将我们的结果与可用的实验数据联系起来。我们的模拟有助于进一步了解谷氨酸变位酶如何催化谷氨酸的碳骨架重排。

































 京公网安备 11010802027423号
京公网安备 11010802027423号