当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
(E)‐3‐(Alkoxycarbonyl‐2‐Alkyliden)‐2‐Oxindoles: Multidentate Pronucleophiles for the Organocatalytic, Vinylogous Michael Addition to Nitroolefins
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-12 , DOI: 10.1002/adsc.201701164
Claudio Curti 1 , Lucia Battistini 1 , Andrea Sartori 1 , Gloria Rassu 2 , Giorgio Pelosi 3 , Marco Lombardo 4 , Franca Zanardi 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-12 , DOI: 10.1002/adsc.201701164
Claudio Curti 1 , Lucia Battistini 1 , Andrea Sartori 1 , Gloria Rassu 2 , Giorgio Pelosi 3 , Marco Lombardo 4 , Franca Zanardi 1
Affiliation
|
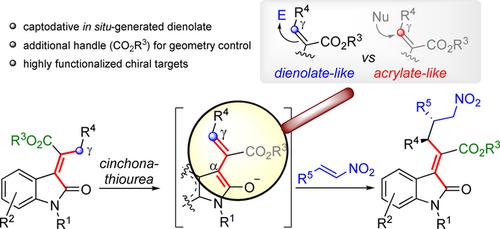
We introduce 3‐(alkoxycarbonyl‐2‐alkyliden)‐2‐oxindoles as pronucleophilic donors in the direct, vinylogous Michael addition to nitroolefins orchestrated by a chiral, bifunctional cinchona‐thiourea organocatalyst. This reaction displays excellent levels of γ‐site‐, diastereo‐ and enantioselectivity delivering valuable enantioenriched functionalized oxindoles. Of note, the C‐γ enolization of these pronucleophiles by the organocatalyst generates a multidentate, captodative dienolate that delivers vinylogous adducts with an unprecedented Z‐selectivity through a peculiar interaction with the catalyst and the nitroolefin. The optimized procedure is operatively simple: the reaction is conducted in air, at room temperature, with low catalyst loading (up to 1 mol%). The synthetic versatility of these Michael adducts is demonstrated by several transformations leading to a valuable quaternary oxindolyl proline analogue and a chiral spirocyclic furoindolone structure. Finally, a mechanistic rationale and a suitable transition state accounting for the observed selectivities are proposed, which are supported by DFT calculations.
中文翻译:

(E)-3-(烷氧羰基-2-烷基)-2-Oxindoles:多齿前亲核体,用于硝基烯烃的有机催化,乙烯基迈克尔加成
我们在手性,双官能金鸡纳硫脲有机催化剂精心策划的硝基烯烃的乙烯基直链乙烯基加成反应中,引入3-(烷氧基羰基-2-烷基)-2-氧吲哚作为亲核供体。此反应显示出极佳的γ-位,非对映和对映选择性,可提供有价值的对映体富集的功能化吲哚。值得注意的是,有机催化剂将这些前亲核体的C-γ烯化反应生成了多齿,Capddative二烯酸酯,可提供具有前所未有Z的乙烯基加合物通过与催化剂和硝基烯烃的特殊相互作用实现选择性。优化的程序操作简单:该反应在空气中,室温下以低催化剂负载量(最高1 mol%)进行。这些迈克尔加合物的合成多功能性通过几次转化得到证实,这些转化导致了有价值的季奥辛多尔脯氨酸类似物和手性螺环呋喃多酮结构。最后,提出了一种机械原理和适合于所观察到的选择性的过渡态,这得到了DFT计算的支持。
更新日期:2017-12-12
中文翻译:

(E)-3-(烷氧羰基-2-烷基)-2-Oxindoles:多齿前亲核体,用于硝基烯烃的有机催化,乙烯基迈克尔加成
我们在手性,双官能金鸡纳硫脲有机催化剂精心策划的硝基烯烃的乙烯基直链乙烯基加成反应中,引入3-(烷氧基羰基-2-烷基)-2-氧吲哚作为亲核供体。此反应显示出极佳的γ-位,非对映和对映选择性,可提供有价值的对映体富集的功能化吲哚。值得注意的是,有机催化剂将这些前亲核体的C-γ烯化反应生成了多齿,Capddative二烯酸酯,可提供具有前所未有Z的乙烯基加合物通过与催化剂和硝基烯烃的特殊相互作用实现选择性。优化的程序操作简单:该反应在空气中,室温下以低催化剂负载量(最高1 mol%)进行。这些迈克尔加合物的合成多功能性通过几次转化得到证实,这些转化导致了有价值的季奥辛多尔脯氨酸类似物和手性螺环呋喃多酮结构。最后,提出了一种机械原理和适合于所观察到的选择性的过渡态,这得到了DFT计算的支持。

































 京公网安备 11010802027423号
京公网安备 11010802027423号