当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Catalytic Asymmetric Synthesis of 3-Hydroxy-3-trifluoromethyl Benzofuranones via Tandem Friedel–Crafts/Lactonization Reaction
Organic Letters ( IF 4.9 ) Pub Date : 2015-09-24 00:00:00 , DOI: 10.1021/acs.orglett.5b02440 Hai Ren 1 , Pan Wang 1 , Lijia Wang 1 , Yong Tang 1
Organic Letters ( IF 4.9 ) Pub Date : 2015-09-24 00:00:00 , DOI: 10.1021/acs.orglett.5b02440 Hai Ren 1 , Pan Wang 1 , Lijia Wang 1 , Yong Tang 1
Affiliation
|
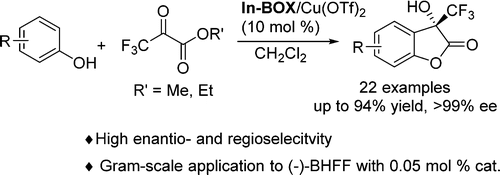
A highly enantioselective and regioselective chiral Lewis acid catalyzed tandem Friedel–Crafts/lactonization reaction is reported, providing direct access to plenty of 3-hydroxy-3-trifluoromethyl benzofuran-2-ones in up to 94% yields with up to >99% ee. Mechanistic study reveals that the interactions between the phenolic hydroxyl group and trifluoropyruvate are the most likely contributing factor to the high enantio- and regioselectivity. Optically pure (−)-BHFF can be obtained in gram-scale with 0.05 mol % catalyst, demonstrating the potentially utility of this method in medicinal chemistry.
中文翻译:

串联Friedel-Crafts /内酯化反应催化不对称合成3-羟基-3-三氟甲基苯并呋喃酮
据报道,具有高度对映选择性和区域选择性的手性路易斯酸催化串联Friedel-Crafts /内酯化反应,可直接获得大量3-羟基-3-三氟甲基苯并呋喃-2-酮,产率高达94%,ee高达99%以上。 。机理研究表明,酚羟基与三氟丙酮酸之间的相互作用是高对映选择性和区域选择性的最可能的因素。可以使用0.05 mol%的催化剂以克为单位获得光学纯的(-)-BHFF,这证明了该方法在药物化学中的潜在用途。
更新日期:2015-09-24
中文翻译:

串联Friedel-Crafts /内酯化反应催化不对称合成3-羟基-3-三氟甲基苯并呋喃酮
据报道,具有高度对映选择性和区域选择性的手性路易斯酸催化串联Friedel-Crafts /内酯化反应,可直接获得大量3-羟基-3-三氟甲基苯并呋喃-2-酮,产率高达94%,ee高达99%以上。 。机理研究表明,酚羟基与三氟丙酮酸之间的相互作用是高对映选择性和区域选择性的最可能的因素。可以使用0.05 mol%的催化剂以克为单位获得光学纯的(-)-BHFF,这证明了该方法在药物化学中的潜在用途。






























 京公网安备 11010802027423号
京公网安备 11010802027423号