当前位置:
X-MOL 学术
›
Adv. Synth. Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
BF3⋅OEt2‐Promoted Annulation for Substituted 2‐Arylpyridines as Potent UV Filters and Antibacterial Agents
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-18 , DOI: 10.1002/adsc.201701137
Sabera Sultana 1 , Shizuka Mei Bautista Maezono 1 , Muhammad Saeed Akhtar 1 , Jae-Jin Shim 1 , Young-Jung Wee 2 , Sung Hong Kim 3 , Yong Rok Lee 1
Advanced Synthesis & Catalysis ( IF 4.4 ) Pub Date : 2017-12-18 , DOI: 10.1002/adsc.201701137
Sabera Sultana 1 , Shizuka Mei Bautista Maezono 1 , Muhammad Saeed Akhtar 1 , Jae-Jin Shim 1 , Young-Jung Wee 2 , Sung Hong Kim 3 , Yong Rok Lee 1
Affiliation
|
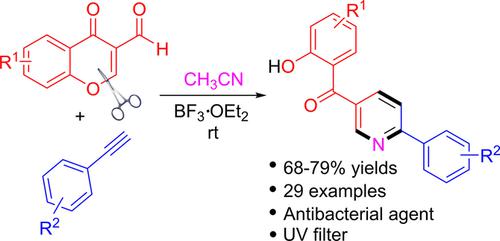
A simple and efficient BF3⋅OEt2 mediated methodology for the construction of diverse 2‐phenylpyridines bearing benzophenone moieties from readily available 3‐formylchromones and phenylacetylenes in wet acetonitrile was developed. The nitrogen source for the pyridine construction was derived from acetonitrile. This one‐pot protocol proceeds via [3+2+1] annulation through cascade nucleophilic addition, hydrolysis, Michael‐type addition, ring opening, and elimination reactions. The synthesized compounds may have applications as UV filters and exhibit potent antibacterial activities.
中文翻译:

BF3⋅OEt2促进的取代2-芳基吡啶作为强紫外线过滤剂和抗菌剂的环
一个简单的,高效的BF 3 ⋅OEt 2为不同的2-苯基吡啶的二苯甲酮轴承部分的从容易获得的3- formylchromones和在湿乙腈phenylacetylenes施工介导的方法学的开发。用于吡啶构建的氮源来自乙腈。这种一锅协议进行经由[3 + 2 + 1]通过级联亲核加成,水解,迈克尔加成,开环,和消除反应环。合成的化合物可以用作紫外线过滤剂并显示出强效的抗菌活性。
更新日期:2017-12-18
中文翻译:

BF3⋅OEt2促进的取代2-芳基吡啶作为强紫外线过滤剂和抗菌剂的环
一个简单的,高效的BF 3 ⋅OEt 2为不同的2-苯基吡啶的二苯甲酮轴承部分的从容易获得的3- formylchromones和在湿乙腈phenylacetylenes施工介导的方法学的开发。用于吡啶构建的氮源来自乙腈。这种一锅协议进行经由[3 + 2 + 1]通过级联亲核加成,水解,迈克尔加成,开环,和消除反应环。合成的化合物可以用作紫外线过滤剂并显示出强效的抗菌活性。































 京公网安备 11010802027423号
京公网安备 11010802027423号