当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Computational Exploration of Concerted and Zwitterionic Mechanisms of Diels–Alder Reactions between 1,2,3-Triazines and Enamines and Acceleration by Hydrogen-Bonding Solvents
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-05 , DOI: 10.1021/jacs.7b08325 Yun-Fang Yang 1 , Peiyuan Yu 1 , K N Houk 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-05 , DOI: 10.1021/jacs.7b08325 Yun-Fang Yang 1 , Peiyuan Yu 1 , K N Houk 1
Affiliation
|
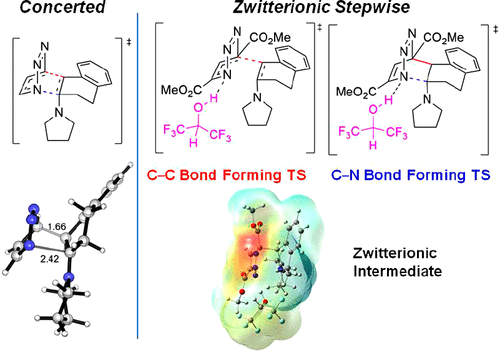
The mechanisms of Diels-Alder reactions between 1,2,3-triazines and enamines have been explored with density functional theory computations. The focus of this work is on the origins of the different reactivities and mechanisms induced by substituents and by hexafluoroisopropanol (HFIP) solvent. These inverse electron-demand Diels-Alder reactions of triazines have wide applications in bioorthogonal chemistry and natural product synthesis. Both concerted and stepwise cycloadditions are predicted, depending on the nature of substituents and solvents. The nature of zwitterionic intermediates and the mechanism by which HFIP accelerates cycloadditions with enamines are characterized. Our results show the delicate nature of the concerted versus stepwise mechanism of inverse electron-demand Diels-Alder reactions of 1,2,3-triazines, and that these mechanisms can be altered by electron-withdrawing substituents and hydrogen-bonding solvents.
中文翻译:

1,2,3-三嗪与烯胺之间 Diels-Alder 反应的协同和两性离子机理的计算探索以及氢键溶剂的加速
通过密度泛函理论计算探索了 1,2,3-三嗪和烯胺之间的 Diels-Alder 反应机理。这项工作的重点是由取代基和六氟异丙醇 (HFIP) 溶剂引起的不同反应性和机制的起源。三嗪的逆电子需求狄尔斯-阿尔德反应在生物正交化学和天然产物合成中具有广泛的应用。根据取代基和溶剂的性质,预计会发生协同环加成和逐步环加成。表征了两性离子中间体的性质以及 HFIP 加速烯胺环加成的机制。我们的结果表明,1,2,3-三嗪的逆电子需求狄尔斯-阿尔德反应的协同机制与逐步机制的微妙性质,并且这些机制可以通过吸电子取代基和氢键溶剂来改变。
更新日期:2017-12-05
中文翻译:

1,2,3-三嗪与烯胺之间 Diels-Alder 反应的协同和两性离子机理的计算探索以及氢键溶剂的加速
通过密度泛函理论计算探索了 1,2,3-三嗪和烯胺之间的 Diels-Alder 反应机理。这项工作的重点是由取代基和六氟异丙醇 (HFIP) 溶剂引起的不同反应性和机制的起源。三嗪的逆电子需求狄尔斯-阿尔德反应在生物正交化学和天然产物合成中具有广泛的应用。根据取代基和溶剂的性质,预计会发生协同环加成和逐步环加成。表征了两性离子中间体的性质以及 HFIP 加速烯胺环加成的机制。我们的结果表明,1,2,3-三嗪的逆电子需求狄尔斯-阿尔德反应的协同机制与逐步机制的微妙性质,并且这些机制可以通过吸电子取代基和氢键溶剂来改变。































 京公网安备 11010802027423号
京公网安备 11010802027423号