当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Laves相LnMg 2(Ln = La,Ce,Pr,Nd,Sm,Eu,Gd,Tb,Ho,Er,Tm,Yb)的氢化性质
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02319
Anton Werwein 1 , Florian Maaß 1 , Leonhard Y. Dorsch 1 , Oliver Janka 2 , Rainer Pöttgen 2 , Thomas C. Hansen 3 , Justin Kimpton 4 , Holger Kohlmann 1
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-22 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02319
Anton Werwein 1 , Florian Maaß 1 , Leonhard Y. Dorsch 1 , Oliver Janka 2 , Rainer Pöttgen 2 , Thomas C. Hansen 3 , Justin Kimpton 4 , Holger Kohlmann 1
Affiliation
|
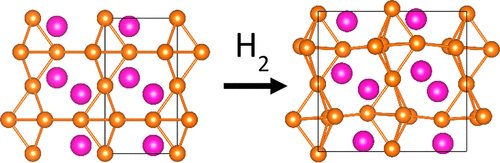
通过热分析,X射线,同步加速器和中子粉末研究了Laves相LnMg 2(Ln = La,Ce,Pr,Nd,Sm,Eu,Gd,Tb,Ho,Er,Tm,Yb)的氢化性质衍射。在14.0兆帕的氢气压力和393 K,PrMg 2和NdMg 2占用氢和形成无色的,三元的氢化物PrMg 2 ħ 7(P 4 1 2 1 2,一个= 632.386(6)时,C ^ = 945.722(11 )pm)和NdMg 2 H 7(P 4 1 2 1 2 a = 630.354(9)pm,c= 943.018(16)pm)。晶体结构通过Rietveld方法从氘核上的中子粉末衍射数据(PrMg 2 D 7,P 4 1 2 1 2 a = 630.56(2)pm,c = 943.27(3)pm; NdMg 2 D 7,P 4 1 2 1 2,a = 628.15(2)pm,c = 940.32(3)pm),显示与LaMg 2 D 7同型。LaMg 2 D 7氢化物类型分别在695 K(La),684 K(Ce),684 K(Pr),672 K(Nd)和639 K(Sm)分解为镧系元素氢化物和镁。Laves相EuMg 2形成黑色的氢化物EuMg 2 H x。它的晶体结构(P 2 1 2 1 2 1,a = 664.887(4)pm,b = 1136.993(7)pm,c = 1069.887(7)pm)与六方Laves相(MgZn 2型)密切相关。无氢母体金属间化合物。GdMg 2和TbMg 2与正交晶胞形成氢化物GdMg 2 H x(a = 1282.7(4)pm,b = 572.5(2)pm,c = 881.7(2)pm)和TbMg 2 H x(a = 617.8(3)pm,b = 1045.8(8)pm,c = 997.1( 5)pm),大概也具有扭曲的MgZn 2型结构。CeMg 2 ħ 7和NdMg 2 ħ 7是顺磁性与μ为2.49(1)有效磁矩乙和3.62(1)μ乙分别,与自由三价稀土阳离子的计算磁矩一致(μ钙(Ce 3+)= 2.54μB ; μ计算值(ND 3+)= 3.62μ乙)。

"点击查看英文标题和摘要"
更新日期:2017-11-22

"点击查看英文标题和摘要"

































 京公网安备 11010802027423号
京公网安备 11010802027423号