Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
The Promise and Challenges of CAR-T Gene Therapy
JAMA ( IF 63.1 ) Pub Date : 2017-12-12 , DOI: 10.1001/jama.2017.15605 Bridget M. Kuehn
JAMA ( IF 63.1 ) Pub Date : 2017-12-12 , DOI: 10.1001/jama.2017.15605 Bridget M. Kuehn
|
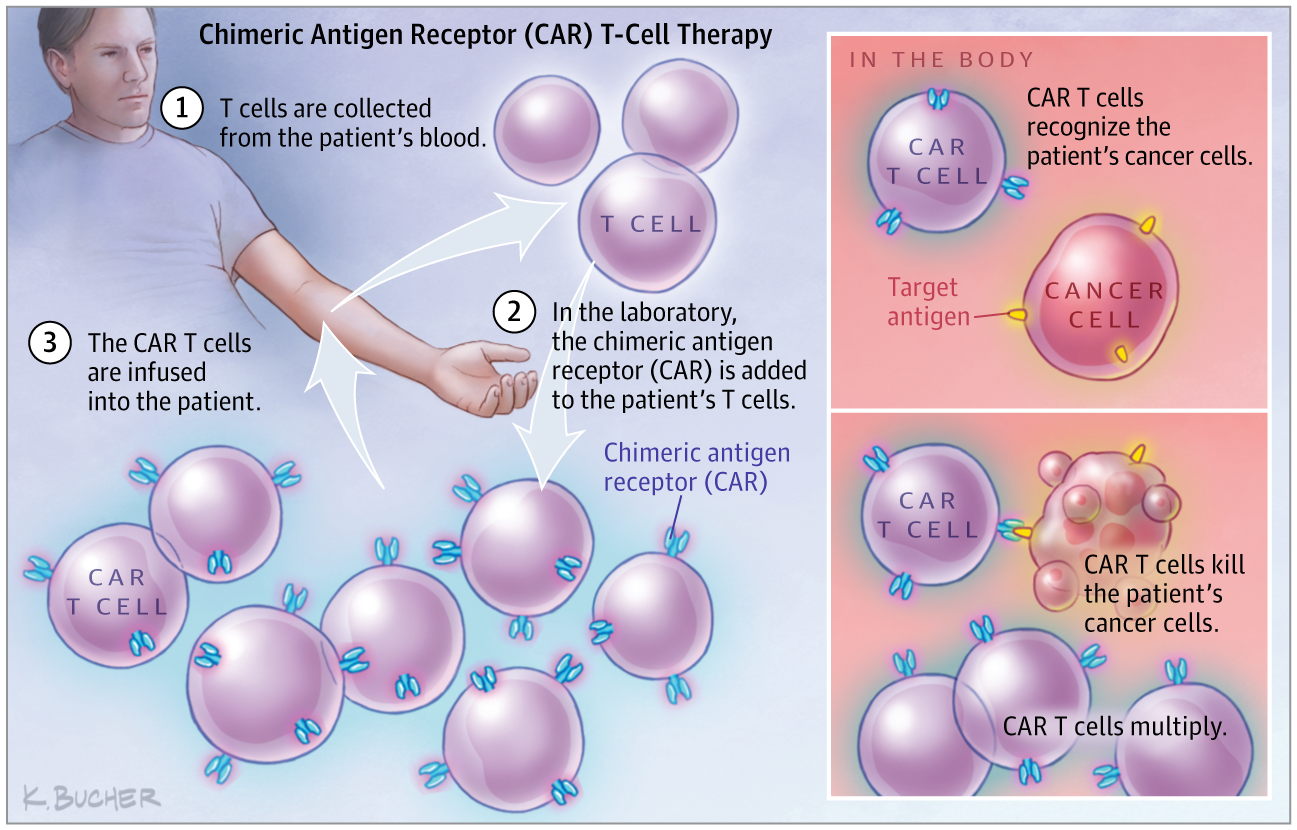
The recent approvals of 2 one-time gene therapies for refractory pediatric acute lymphoblastic leukemia (ALL) and adult relapsed or refractory diffuse large B-cell lymphoma mark a milestone for gene therapy and may open up a new treatment option for thousands of patients for whom traditional cancer therapies have failed. In August, the US Food and Drug Administration (FDA) approved tisagenlecleucel, a chimeric antigen receptor T-cell (CAR T-cell) therapy for the treatment of patients with refractory B-cell precursor ALL who are up to 25 years old. About 3100 patients a year develop B-cell precursor ALL, and 15% to 20% do not respond to or experience a relapse after traditional therapies, according to the FDA. This treatment is indicated for the subset of this group who have been unsuccessfully treated with other therapies more than once. In the therapy, a patient’s own T cells are removed and genetically engineered to recognize and destroy leukemia cells that have the CD19 antigen on their cell surface. Then, the cells are infused back into the patient. The treatment will be offered at about 30 centers. “The [tisagenlecleucel] approval was an important first step,” said David Porter, MD, a leukemia specialist and director of the Blood and Bone Marrow Center at the University of Pennsylvania who helped lead the therapy’s trial. “It opens up the ability for many more young people with relapsed and refractory ALL to have access to a potentially life-saving treatment.” A few months later, axicabtagene ciloleucel, a similar CAR-T therapy for adults with relapsed or refractory diffuse large B-cell lymphoma, became the second FDA-approved gene therapy. About 7500 patients a year may be eligible for this treatment, according to Gilead Sciences, which recently purchased the drug’s maker Kite. The drug is the fruit of decades of work developing cell-based therapies by researchers at the National Institutes of Health, according to Steve Rosenberg, MD, PhD, chief of the surgery branch at the National Cancer Institute. “For me, this has been a very long journey, but finally we are seeing these highly personalized cell therapies using genetically engineered cells be in a position to help patients in need,” said Rosenberg. “It’s a very exciting time.” These approvals mark a turning point for the field, said Rosenberg. Initially, axicabtagene ciloleucel will be offered at 16 centers, but the drug’s maker said it has plans to eventually expand to 90. Although these achievements are impressive, some experts caution that hurdles—including serious adverse events and high costs—remain before wider application of these therapies.
中文翻译:

CAR-T基因治疗的前景与挑战
最近批准用于难治性小儿急性淋巴细胞白血病 (ALL) 和成人复发或难治性弥漫性大 B 细胞淋巴瘤的 2 种一次性基因疗法标志着基因治疗的一个里程碑,并可能为数千名患者开辟新的治疗选择传统的癌症疗法已经失败。8 月,美国食品和药物管理局 (FDA) 批准了 tisagenlecleucel,一种嵌合抗原受体 T 细胞(CAR T 细胞)疗法,用于治疗 25 岁以下难治性 B 细胞前体 ALL 患者。据 FDA 称,每年约有 3100 名患者发展为 B 细胞前体 ALL,15% 至 20% 的患者在传统疗法后没有反应或复发。这种治疗适用于该组中多次用其他疗法治疗失败的亚组。在治疗中,患者自身的 T 细胞被移除并进行基因工程改造,以识别和破坏细胞表面具有 CD19 抗原的白血病细胞。然后,将细胞输回患者体内。治疗将在大约 30 个中心提供。“[tisagenlecleucel] 的批准是重要的第一步,”宾夕法尼亚大学白血病专家兼血液和骨髓中心主任大卫波特说,他帮助领导了该疗法的试验。“它为更多患有复发性和难治性 ALL 的年轻人提供了获得可能挽救生命的治疗的能力。” 几个月后,axicabtagene ciloleucel,一种用于成人复发或难治性弥漫性大 B 细胞淋巴瘤的类似 CAR-T 疗法,成为 FDA 批准的第二个基因疗法。据最近收购了该药物制造商 Kite 的吉利德科学公司称,每年约有 7500 名患者可能有资格接受这种治疗。据美国国家癌症研究所外科部门负责人史蒂夫·罗森伯格 (Steve Rosenberg) 医学博士称,该药物是美国国立卫生研究院研究人员数十年开发基于细胞疗法的成果。“对我来说,这是一段漫长的旅程,但最终我们看到这些使用基因工程细胞的高度个性化细胞疗法能够帮助有需要的患者,”罗森伯格说。“这是一个非常激动人心的时刻。” Rosenberg 说,这些批准标志着该领域的一个转折点。最初,axicabtagene ciloleucel 将在 16 个中心提供,但该药物制造商表示,它计划最终扩展到 90 个。尽管这些成就令人印象深刻,
更新日期:2017-12-12
中文翻译:

CAR-T基因治疗的前景与挑战
最近批准用于难治性小儿急性淋巴细胞白血病 (ALL) 和成人复发或难治性弥漫性大 B 细胞淋巴瘤的 2 种一次性基因疗法标志着基因治疗的一个里程碑,并可能为数千名患者开辟新的治疗选择传统的癌症疗法已经失败。8 月,美国食品和药物管理局 (FDA) 批准了 tisagenlecleucel,一种嵌合抗原受体 T 细胞(CAR T 细胞)疗法,用于治疗 25 岁以下难治性 B 细胞前体 ALL 患者。据 FDA 称,每年约有 3100 名患者发展为 B 细胞前体 ALL,15% 至 20% 的患者在传统疗法后没有反应或复发。这种治疗适用于该组中多次用其他疗法治疗失败的亚组。在治疗中,患者自身的 T 细胞被移除并进行基因工程改造,以识别和破坏细胞表面具有 CD19 抗原的白血病细胞。然后,将细胞输回患者体内。治疗将在大约 30 个中心提供。“[tisagenlecleucel] 的批准是重要的第一步,”宾夕法尼亚大学白血病专家兼血液和骨髓中心主任大卫波特说,他帮助领导了该疗法的试验。“它为更多患有复发性和难治性 ALL 的年轻人提供了获得可能挽救生命的治疗的能力。” 几个月后,axicabtagene ciloleucel,一种用于成人复发或难治性弥漫性大 B 细胞淋巴瘤的类似 CAR-T 疗法,成为 FDA 批准的第二个基因疗法。据最近收购了该药物制造商 Kite 的吉利德科学公司称,每年约有 7500 名患者可能有资格接受这种治疗。据美国国家癌症研究所外科部门负责人史蒂夫·罗森伯格 (Steve Rosenberg) 医学博士称,该药物是美国国立卫生研究院研究人员数十年开发基于细胞疗法的成果。“对我来说,这是一段漫长的旅程,但最终我们看到这些使用基因工程细胞的高度个性化细胞疗法能够帮助有需要的患者,”罗森伯格说。“这是一个非常激动人心的时刻。” Rosenberg 说,这些批准标志着该领域的一个转折点。最初,axicabtagene ciloleucel 将在 16 个中心提供,但该药物制造商表示,它计划最终扩展到 90 个。尽管这些成就令人印象深刻,






































 京公网安备 11010802027423号
京公网安备 11010802027423号