JAMA ( IF 63.1 ) Pub Date : 2017-11-21 , DOI: 10.1001/jama.2017.17384
|
Pancreatic enzyme replacement products have been used for years to improve digestion in patients with exocrine pancreatic insufficiency (EPI). These products were initially marketed before formal FDA approval was required; in 1991, however, the FDA told all manufacturers of pancreatic enzyme replacement products that they would have to submit a new drug application by April 2010 in order to keep their products on the market.
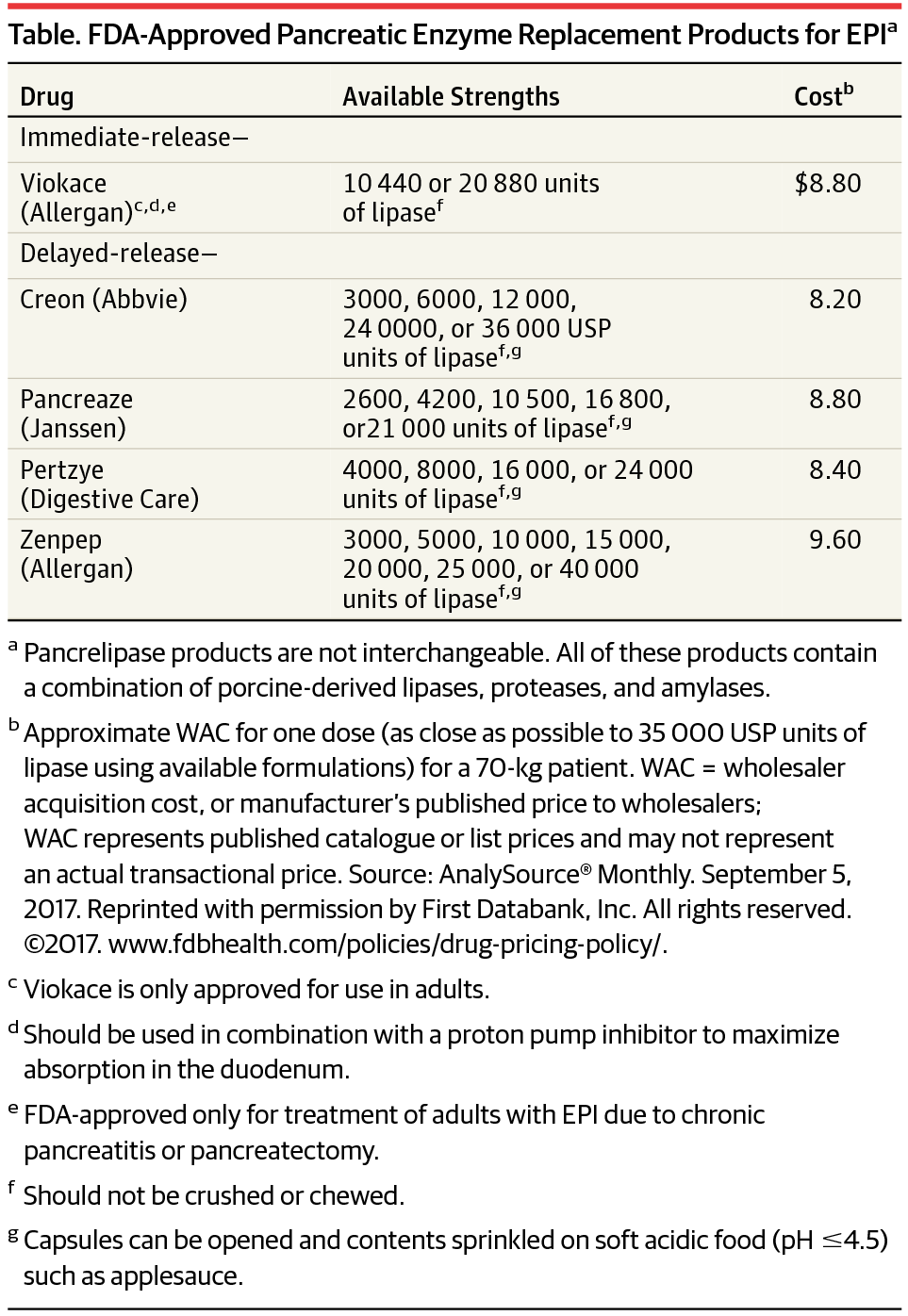
EPI is a chronic disorder characterized by a deficiency of exocrine pancreatic enzymes, which results in malabsorption, steatorrhea, and weight loss. Irreversible EPI is commonly caused by chronic pancreatitis in adults and by cystic fibrosis in children. The standard treatment for EPI is pancreatic enzyme replacement therapy (Table). Treatment with pancreatic enzyme replacement products has reduced fat malabsorption and steatorrhea.1
中文翻译:

胰酶替代产品
胰腺酶替代产品已用于改善外分泌型胰腺功能不全(EPI)患者的消化能力多年。这些产品最初是在需要正式的FDA批准之前销售的;然而,在1991年,FDA告知所有胰腺酶替代产品制造商,他们必须在2010年4月之前提交新药申请,以使其产品保持在市场上。
EPI是一种慢性疾病,其特征是外分泌胰腺酶缺乏,导致吸收不良,脂肪泻和体重减轻。不可逆的EPI通常由成人慢性胰腺炎和儿童囊性纤维化引起。EPI的标准治疗方法是胰酶替代治疗(表)。用胰酶替代产品治疗可减少脂肪吸收不良和脂肪泻。1个































 京公网安备 11010802027423号
京公网安备 11010802027423号