当前位置:
X-MOL 学术
›
J. Phys. Chem. B
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mechanism of Competitive Inhibition and Destabilization of Acidothermus cellulolyticus Endoglucanase 1 by Ionic Liquids
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b08435
Samantha R. Summers 1 , K. G. Sprenger 2 , Jim Pfaendtner 2 , Jan Marchant 3 , Michael F. Summers 3 , Joel L. Kaar 1
The Journal of Physical Chemistry B ( IF 2.8 ) Pub Date : 2017-11-21 00:00:00 , DOI: 10.1021/acs.jpcb.7b08435
Samantha R. Summers 1 , K. G. Sprenger 2 , Jim Pfaendtner 2 , Jan Marchant 3 , Michael F. Summers 3 , Joel L. Kaar 1
Affiliation
|
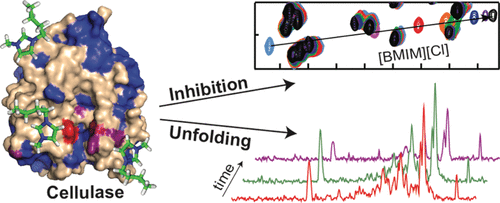
The ability of ionic liquids (ILs) to solubilize cellulose has sparked interest in their use for enzymatic biomass processing. However, this potential is yet to be realized, primarily because ILs inactivate requisite cellulases by mechanisms that are yet to be identified. We used a combination of enzymology, circular dichroism (CD), nuclear magnetic resonance (NMR), and molecular dynamics (MD) methods to investigate the molecular basis for the inactivation of the endocellulase 1 (E1) from Acidothermus cellulolyticus by the imidazolium IL 1-butyl-3-methylimidazolium chloride ([BMIM][Cl]). Enzymatic studies revealed that [BMIM][Cl] inactivates E1 in a biphasic manner that involves rapid, reversible inhibition, followed by slow, irreversible deactivation. Backbone NMR signals of the 40.5 kDa E1 were assigned by triple resonance NMR methods, enabling monitoring of residue-specific perturbations. 1H–15N NMR titration experiments revealed that [BMIM][Cl] binds reversibly to the E1 active site, indicating that reversible deactivation is due to competitive inhibition of substrate binding. Prolonged incubation with [BMIM][Cl] led to substantial global changes in the 1H–15N heteronuclear single quantum coherence NMR and CD spectra of E1 indicative of protein denaturation. Notably, weak interactions between [BMIM][Cl] and residues at the termini of several helices were also observed, which, together with MD simulations, suggest that E1 denaturation is promoted by [BMIM][Cl]-induced destabilization of helix capping structures. In addition to identifying determinants of E1 inactivation, our findings establish a molecular framework for engineering cellulases with improved IL compatibility.
中文翻译:

离子液体竞争抑制和解热解酸纤维素酶内切葡聚糖酶的机理。
离子液体(ILs)溶解纤维素的能力引起了人们对其在酶促生物质加工中的应用的兴趣。但是,这种潜力尚未实现,主要是因为IL通过尚未确定的机制使必需的纤维素酶失活。我们结合使用了酶学,圆二色性(CD),核磁共振(NMR)和分子动力学(MD)方法,研究了解酸嗜酸菌中内切酶1(E1)失活的分子基础。通过咪唑鎓盐IL 1-丁基-3-甲基咪唑鎓氯化物([BMIM] [Cl])。酶学研究表明,[BMIM] [Cl]以双相方式使E1失活,涉及快速,可逆的抑制,然后是缓慢的,不可逆的失活。通过三重共振NMR方法分配了40.5 kDa E1的主干NMR信号,从而可以监测残基特异性扰动。1 H– 15 N NMR滴定实验表明[BMIM] [Cl]可逆地结合到E1活性位点,表明可逆的失活是由于竞争性抑制底物结合所致。与[BMIM] [Cl]的长时间孵育导致1 H– 15的整体变化E1的N异核单量子相干NMR和CD光谱指示蛋白质变性。值得注意的是,还观察到[BMIM] [Cl]与几个螺旋末端的残基之间的弱相互作用,这与MD模拟一起表明,[BMIM] [Cl]引起的螺旋帽结构的失稳促进了E1变性。 。除了确定E1失活的决定因素外,我们的发现还建立了工程纤维素酶具有改善的IL相容性的分子框架。
更新日期:2017-11-22
中文翻译:

离子液体竞争抑制和解热解酸纤维素酶内切葡聚糖酶的机理。
离子液体(ILs)溶解纤维素的能力引起了人们对其在酶促生物质加工中的应用的兴趣。但是,这种潜力尚未实现,主要是因为IL通过尚未确定的机制使必需的纤维素酶失活。我们结合使用了酶学,圆二色性(CD),核磁共振(NMR)和分子动力学(MD)方法,研究了解酸嗜酸菌中内切酶1(E1)失活的分子基础。通过咪唑鎓盐IL 1-丁基-3-甲基咪唑鎓氯化物([BMIM] [Cl])。酶学研究表明,[BMIM] [Cl]以双相方式使E1失活,涉及快速,可逆的抑制,然后是缓慢的,不可逆的失活。通过三重共振NMR方法分配了40.5 kDa E1的主干NMR信号,从而可以监测残基特异性扰动。1 H– 15 N NMR滴定实验表明[BMIM] [Cl]可逆地结合到E1活性位点,表明可逆的失活是由于竞争性抑制底物结合所致。与[BMIM] [Cl]的长时间孵育导致1 H– 15的整体变化E1的N异核单量子相干NMR和CD光谱指示蛋白质变性。值得注意的是,还观察到[BMIM] [Cl]与几个螺旋末端的残基之间的弱相互作用,这与MD模拟一起表明,[BMIM] [Cl]引起的螺旋帽结构的失稳促进了E1变性。 。除了确定E1失活的决定因素外,我们的发现还建立了工程纤维素酶具有改善的IL相容性的分子框架。







































 京公网安备 11010802027423号
京公网安备 11010802027423号