当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
1,3,5‐Oxadiazine Framework by Oxygen vs. Nitrogen Trapping of an N‐Acyliminium Ion Derived from N,O‐bis‐TMS Pyroglutamic Acid
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-11-22 , DOI: 10.1002/slct.201701766
Alina Ghinet 1, 2, 3 , Cristina-Maria Abuhaie 1, 2, 3 , Germain Homerin 1, 2 , Hamid Marzag 4 , Joëlle Dubois 5 , Emmanuelle Lipka 2, 6 , Benoît Rigo 1, 2 , Adam Daïch 4
ChemistrySelect ( IF 1.9 ) Pub Date : 2017-11-22 , DOI: 10.1002/slct.201701766
Alina Ghinet 1, 2, 3 , Cristina-Maria Abuhaie 1, 2, 3 , Germain Homerin 1, 2 , Hamid Marzag 4 , Joëlle Dubois 5 , Emmanuelle Lipka 2, 6 , Benoît Rigo 1, 2 , Adam Daïch 4
Affiliation
|
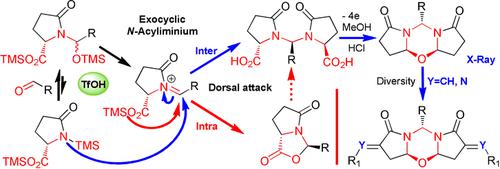
We report that the reaction of N,O‐bis‐TMS pyroglutamic acid with aldehydes, under basic or acid catalysis leads to O‐bis‐TMS adducts. Treatment of these N,O‐acetals by TfOH affords azalactones or N,N′‐substituted methylene‐bis‐pyroglutamic acids from the trapping of N‐acyliminium species, respectively, with oxygen or nitrogen atom intramolecularly versus intermolecularly. Anodic oxidation of the amido acids, followed by diastereoselective oxa‐cyclisation of new N‐acyliminium salts, provides exclusively fused meso‐1,3,5‐oxadiazines with the stereochemistry secured by X‐ray analysis. The reactivity of these skeletons under both alkaline and acid conditions was also envisioned and discussed.
中文翻译:

N,O-bis-TMS焦谷氨酸衍生的N-酰基亚胺离子的1,3,5-Oxadiazine骨架的氧与氮截留
我们报告说,在碱性或酸催化下,N,O -bis-TMS焦谷氨酸与醛的反应会导致O -bis-TMS加合物。用TfOH处理这些N,O-乙缩醛可分别从N-酰基亚胺类化合物的分子或分子内与氧或氮原子的捕获中获得氮杂内酯或N,N'-取代的亚甲基双焦谷氨酸。酰胺酸的阳极氧化,然后对新氮进行非对映选择性的氧杂环化氰基亚胺盐专门提供熔融的meso-1,3,5-恶二嗪,其立体化学由X射线分析确定。还设想并讨论了这些骨架在碱性和酸性条件下的反应性。
更新日期:2017-11-22
中文翻译:

N,O-bis-TMS焦谷氨酸衍生的N-酰基亚胺离子的1,3,5-Oxadiazine骨架的氧与氮截留
我们报告说,在碱性或酸催化下,N,O -bis-TMS焦谷氨酸与醛的反应会导致O -bis-TMS加合物。用TfOH处理这些N,O-乙缩醛可分别从N-酰基亚胺类化合物的分子或分子内与氧或氮原子的捕获中获得氮杂内酯或N,N'-取代的亚甲基双焦谷氨酸。酰胺酸的阳极氧化,然后对新氮进行非对映选择性的氧杂环化氰基亚胺盐专门提供熔融的meso-1,3,5-恶二嗪,其立体化学由X射线分析确定。还设想并讨论了这些骨架在碱性和酸性条件下的反应性。

































 京公网安备 11010802027423号
京公网安备 11010802027423号