当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Control of the Switching Speed of Photochromic Naphthopyrans through Restriction of Double Bond Isomerization
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.joc.7b01669 Céu M. Sousa 1 , Jerome Berthet 2 , Stephanie Delbaere 2 , André Polónia 1 , Paulo J. Coelho 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-20 00:00:00 , DOI: 10.1021/acs.joc.7b01669 Céu M. Sousa 1 , Jerome Berthet 2 , Stephanie Delbaere 2 , André Polónia 1 , Paulo J. Coelho 1
Affiliation
|
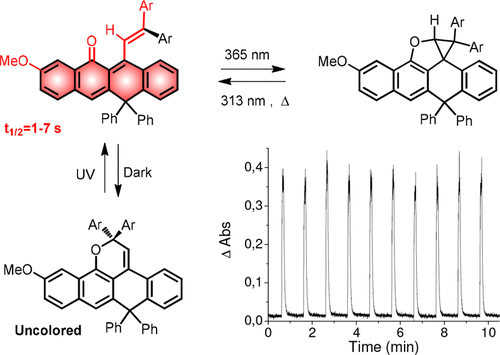
An efficient synthesis of photochromic fused-naphthopyrans was developed. UV–vis or sunlight irradiation of these uncolored compounds in solution led to the formation of a single colored photoisomer along with an unusual and uncolored bicyclic compound formed through an intramolecular photochemical Diels–Alder reaction. Both species faded thermally in the dark to the initial form. A mechanism for this transformation is proposed based on NMR studies of irradiated solutions. The new fused-naphthopyrans have been incorporated into hybrid organic–inorganic matrices affording light-yellow materials that develop intense red colorations under UV light and return to the initial uncolored state in just a few seconds, in the dark, at room temperature. These results are useful for the development of fast switching materials used in the production of photochromic lenses.
中文翻译:

通过限制双键异构化控制光致变色萘并吡喃的转换速度
开发了一种光致变色稠合萘并吡喃的有效合成方法。溶液中这些未着色化合物的紫外可见光或日光照射导致形成了一种单一的有色光异构体,以及通过分子内光化学Diels-Alder反应形成的一种不寻常且未着色的双环化合物。两种物种都在黑暗中热褪色至初始形式。基于辐照溶液的NMR研究,提出了这种转化的机理。新的稠合萘并吡喃已被掺入有机-无机杂化基质中,提供浅黄色材料,它们在紫外光下会呈现强烈的红色,并在黑暗中于室温下在短短几秒钟内恢复到初始的无色状态。
更新日期:2017-11-21
中文翻译:

通过限制双键异构化控制光致变色萘并吡喃的转换速度
开发了一种光致变色稠合萘并吡喃的有效合成方法。溶液中这些未着色化合物的紫外可见光或日光照射导致形成了一种单一的有色光异构体,以及通过分子内光化学Diels-Alder反应形成的一种不寻常且未着色的双环化合物。两种物种都在黑暗中热褪色至初始形式。基于辐照溶液的NMR研究,提出了这种转化的机理。新的稠合萘并吡喃已被掺入有机-无机杂化基质中,提供浅黄色材料,它们在紫外光下会呈现强烈的红色,并在黑暗中于室温下在短短几秒钟内恢复到初始的无色状态。

































 京公网安备 11010802027423号
京公网安备 11010802027423号