当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Peramivir analogues bearing hydrophilic side chains exhibit higher activities against H275Y mutant than wild-type influenza virus
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7ob02374j Din-Chi Chiu,Tzu-Chen Lin,Wen-I Huang,Ting-Jen Cheng,Keng-Chang Tsai,Jim-Min Fang
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2017-11-09 00:00:00 , DOI: 10.1039/c7ob02374j Din-Chi Chiu,Tzu-Chen Lin,Wen-I Huang,Ting-Jen Cheng,Keng-Chang Tsai,Jim-Min Fang
|
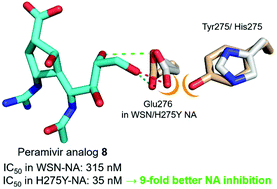
Peramivir is an effective anti-influenza drug in the clinical treatment of influenza, but its efficacy toward the H275Y mutant is reduced. The previously reported cocrystal structures of inhibitors in the mutant neuraminidase (NA) suggest that the hydrophobic side chain should be at the origin of reduced binding affinity. In contrast, zanamivir having a hydrophilic glycerol side chain still possesses high affinity toward the H275Y NA. We thus designed five peramivir analogues (5–9) carrying hydrophilic glycol or glycerol side chains, and evaluated their roles in anti-influenza activity, especially for the H275Y mutant. The synthetic sequence involves a key step of (3 + 2) cycloaddition reactions between alkenes and nitrile oxides to construct the scaffold of peramivir carrying the desired hydrophilic side chains and other appropriate functional groups. The molecular docking experiments reveal that the hydrophilic side chain can provide extra hydrogen bonding with the translocated Glu-276 residue in the H275Y NA active site. Thus, the H275Y mutant may be even more sensitive than wild-type virus toward the peramivir analogues bearing hydrophilic side chains. Notably, the peramivir analogue bearing a glycerol side chain inhibits the H275Y mutant with an IC50 value of 35 nM, which is better than the WSN virus by 9 fold.
中文翻译:

带有亲水性侧链的帕拉米韦类似物对H275Y突变体的活性高于野生型流感病毒
帕拉米韦在流感的临床治疗中是一种有效的抗流感药物,但其对H275Y突变体的功效降低。先前报道的突变神经氨酸酶(NA)中抑制剂的共晶体结构表明,疏水性侧链应位于结合亲和力降低的起点。相反,具有亲水性甘油侧链的扎那米韦仍然对H275Y NA具有高亲和力。因此,我们设计了五个peramivir类似物(5–9)带有亲水性乙二醇或甘油侧链,并评估了它们在抗流感活性中的作用,尤其是对于H275Y突变体。合成序列涉及烯烃和腈氧化物之间的(3 + 2)环加成反应的关键步骤,以构建携带所需亲水性侧链和其他适当官能团的帕拉米韦支架。分子对接实验表明,亲水性侧链可以与H275Y NA活性位点中转移的Glu-276残基提供额外的氢键。因此,对于携带亲水性侧链的peramivir类似物,H275Y突变体可能比野生型病毒更敏感。值得注意的是,带有甘油侧链的peramivir类似物具有IC 50抑制H275Y突变体 值是35 nM,比WSN病毒好9倍。
更新日期:2017-11-21
中文翻译:

带有亲水性侧链的帕拉米韦类似物对H275Y突变体的活性高于野生型流感病毒
帕拉米韦在流感的临床治疗中是一种有效的抗流感药物,但其对H275Y突变体的功效降低。先前报道的突变神经氨酸酶(NA)中抑制剂的共晶体结构表明,疏水性侧链应位于结合亲和力降低的起点。相反,具有亲水性甘油侧链的扎那米韦仍然对H275Y NA具有高亲和力。因此,我们设计了五个peramivir类似物(5–9)带有亲水性乙二醇或甘油侧链,并评估了它们在抗流感活性中的作用,尤其是对于H275Y突变体。合成序列涉及烯烃和腈氧化物之间的(3 + 2)环加成反应的关键步骤,以构建携带所需亲水性侧链和其他适当官能团的帕拉米韦支架。分子对接实验表明,亲水性侧链可以与H275Y NA活性位点中转移的Glu-276残基提供额外的氢键。因此,对于携带亲水性侧链的peramivir类似物,H275Y突变体可能比野生型病毒更敏感。值得注意的是,带有甘油侧链的peramivir类似物具有IC 50抑制H275Y突变体 值是35 nM,比WSN病毒好9倍。































 京公网安备 11010802027423号
京公网安备 11010802027423号