当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetics of Silane Decomposition in High-Pressure Confined Chemical Vapor Deposition of Hydrogenated Amorphous Silicon
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acs.iecr.7b03515
Seyed Pouria Motevalian 1 , Stephen C. Aro 1 , Hiu Y. Cheng 1 , Todd D. Day 1 , Adri C. T. van Duin 1 , John V. Badding 1 , Ali Borhan 1
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acs.iecr.7b03515
Seyed Pouria Motevalian 1 , Stephen C. Aro 1 , Hiu Y. Cheng 1 , Todd D. Day 1 , Adri C. T. van Duin 1 , John V. Badding 1 , Ali Borhan 1
Affiliation
|
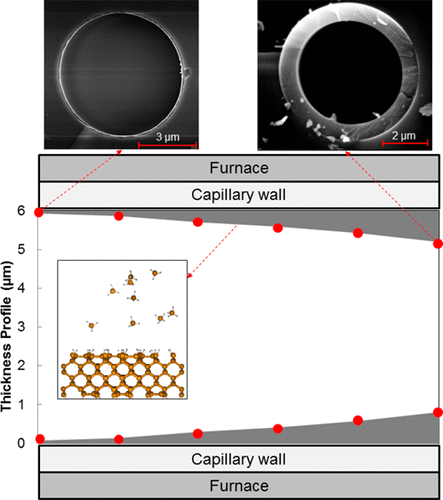
We study the kinetics of silane pyrolysis via confined high-pressure chemical vapor deposition (HPCVD) at pressures of 20–33 MPa in a microcapillary of 5.9 μm inner diameter. We find the growth rate to be first order with respect to silane concentration, with an activation energy of 53.7 ± 2.9 kcal/mol and a pre-exponential factor of 1.5 × 1010 m/s. The obtained activation energy is in the range of activation energies reported for hydrogen desorption from c-Si surfaces, suggesting that hydrogen desorption from the surface is the rate-limiting step in film growth. To further investigate this finding, reactive molecular dynamics simulations of thermal decomposition of silane on clean and hydrogen-passivated c-Si were performed. Homogeneous reactions were not observed in any of the simulations, in support of the hypothesis that heterogeneous silane decomposition on the silicon surface is the dominant mechanism for film deposition. In silane pyrolysis simulations on clean c-Si surfaces, almost all available silicon surface sites (i.e., dangling bonds) were occupied by silicon-hydrides (mostly tri- and dihydrides) upon exposure to gas-phase silane, whereas no reaction was observed during silane decomposition simulations on the hydrogen-passivated c-Si. Therefore, the results of the reactive molecular dynamics simulations indicate that the availability of dangling bonds resulting from hydrogen desorption from the surface is the rate-limiting step in film growth at high pressure.
中文翻译:

氢化非晶硅高压密闭化学气相沉积中硅烷分解的动力学
我们研究了通过内径为5.9μm的微毛细管在20-33 MPa的压力下通过密闭高压化学气相沉积(HPCVD)进行的硅烷热解动力学。我们发现相对于硅烷浓度,生长速率是一阶的,活化能为53.7±2.9 kcal / mol,预指数因子为1.5×10 10多发性硬化症。所获得的活化能处于报道的氢从c-Si表面脱附的活化能的范围内,这表明氢从表面脱附是膜生长中的限速步骤。为了进一步研究这一发现,进行了在干净和氢钝化的c-Si上硅烷热分解的反应性分子动力学模拟。在任何模拟中均未观察到均相反应,以支持以下假设:硅表面上异质硅烷分解是薄膜沉积的主要机理。在干净的c-Si表面进行硅烷热解模拟时,暴露于气相硅烷后,几乎所有可用的硅表面位点(即,悬空键)都被氢化硅(主要是三氢化物和二氢化物)占据,而在氢钝化c-Si的硅烷分解模拟过程中未观察到反应。因此,反应分子动力学模拟的结果表明,由于氢从表面脱附而产生的悬空键是高压下薄膜生长的限速步骤。
更新日期:2017-12-18
中文翻译:

氢化非晶硅高压密闭化学气相沉积中硅烷分解的动力学
我们研究了通过内径为5.9μm的微毛细管在20-33 MPa的压力下通过密闭高压化学气相沉积(HPCVD)进行的硅烷热解动力学。我们发现相对于硅烷浓度,生长速率是一阶的,活化能为53.7±2.9 kcal / mol,预指数因子为1.5×10 10多发性硬化症。所获得的活化能处于报道的氢从c-Si表面脱附的活化能的范围内,这表明氢从表面脱附是膜生长中的限速步骤。为了进一步研究这一发现,进行了在干净和氢钝化的c-Si上硅烷热分解的反应性分子动力学模拟。在任何模拟中均未观察到均相反应,以支持以下假设:硅表面上异质硅烷分解是薄膜沉积的主要机理。在干净的c-Si表面进行硅烷热解模拟时,暴露于气相硅烷后,几乎所有可用的硅表面位点(即,悬空键)都被氢化硅(主要是三氢化物和二氢化物)占据,而在氢钝化c-Si的硅烷分解模拟过程中未观察到反应。因此,反应分子动力学模拟的结果表明,由于氢从表面脱附而产生的悬空键是高压下薄膜生长的限速步骤。

































 京公网安备 11010802027423号
京公网安备 11010802027423号