Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Post-transcriptional Regulation of De Novo Lipogenesis by mTORC1-S6K1-SRPK2 Signaling.
Cell ( IF 45.5 ) Pub Date : 2017-Dec-14 , DOI: 10.1016/j.cell.2017.10.037 Gina Lee , Yuxiang Zheng , Sungyun Cho , Cholsoon Jang , Christina England , Jamie M. Dempsey , Yonghao Yu , Xiaolei Liu , Long He , Paola M. Cavaliere , Andre Chavez , Erik Zhang , Meltem Isik , Anthony Couvillon , Noah E. Dephoure , T. Keith Blackwell , Jane J. Yu , Joshua D. Rabinowitz , Lewis C. Cantley , John Blenis
Cell ( IF 45.5 ) Pub Date : 2017-Dec-14 , DOI: 10.1016/j.cell.2017.10.037 Gina Lee , Yuxiang Zheng , Sungyun Cho , Cholsoon Jang , Christina England , Jamie M. Dempsey , Yonghao Yu , Xiaolei Liu , Long He , Paola M. Cavaliere , Andre Chavez , Erik Zhang , Meltem Isik , Anthony Couvillon , Noah E. Dephoure , T. Keith Blackwell , Jane J. Yu , Joshua D. Rabinowitz , Lewis C. Cantley , John Blenis
|
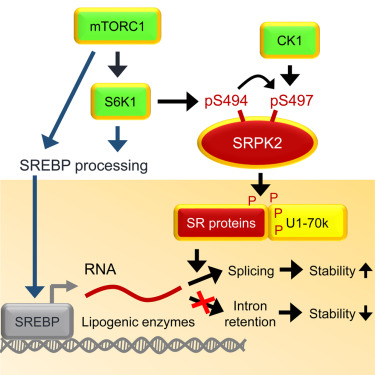
mTORC1 is a signal integrator and master regulator of cellular anabolic processes linked to cell growth and survival. Here, we demonstrate that mTORC1 promotes lipid biogenesis via SRPK2, a key regulator of RNA-binding SR proteins. mTORC1-activated S6K1 phosphorylates SRPK2 at Ser494, which primes Ser497 phosphorylation by CK1. These phosphorylation events promote SRPK2 nuclear translocation and phosphorylation of SR proteins. Genome-wide transcriptome analysis reveals that lipid biosynthetic enzymes are among the downstream targets of mTORC1-SRPK2 signaling. Mechanistically, SRPK2 promotes SR protein binding to U1-70K to induce splicing of lipogenic pre-mRNAs. Inhibition of this signaling pathway leads to intron retention of lipogenic genes, which triggers nonsense-mediated mRNA decay. Genetic or pharmacological inhibition of SRPK2 blunts de novo lipid synthesis, thereby suppressing cell growth. These results thus reveal a novel role of mTORC1-SRPK2 signaling in post-transcriptional regulation of lipid metabolism and demonstrate that SRPK2 is a potential therapeutic target for mTORC1-driven metabolic disorders.
中文翻译:

从头脂代谢的mTORC1-S6K1-SRPK2信号传导的转录后调控。
mTORC1是与细胞生长和存活相关的细胞合成代谢过程的信号整合剂和主调节剂。在这里,我们证明mTORC1通过SRPK2促进脂质生物发生,SRPK2是RNA结合SR蛋白的关键调节剂。mTORC1激活的S6K1使Ser494处的SRPK2磷酸化,从而引发CK1引起的Ser497磷酸化。这些磷酸化事件促进SRPK2核易位和SR蛋白的磷酸化。全基因组转录组分析表明,脂质生物合成酶是mTORC1-SRPK2信号转导的下游目标之一。从机理上讲,SRPK2促进SR蛋白与U1-70K的结合,从而诱导成脂前体mRNA的剪接。该信号通路的抑制导致内含子保留脂肪生成基因,从而触发无意义介导的mRNA衰变。SRPK2钝性从头合成脂质的遗传或药理抑制作用,从而抑制细胞生长。因此,这些结果揭示了mTORC1-SRPK2信号传导在脂质代谢的转录后调控中的新型作用,并证明SRPK2是mTORC1驱动的代谢性疾病的潜在治疗靶标。
更新日期:2017-11-19
中文翻译:

从头脂代谢的mTORC1-S6K1-SRPK2信号传导的转录后调控。
mTORC1是与细胞生长和存活相关的细胞合成代谢过程的信号整合剂和主调节剂。在这里,我们证明mTORC1通过SRPK2促进脂质生物发生,SRPK2是RNA结合SR蛋白的关键调节剂。mTORC1激活的S6K1使Ser494处的SRPK2磷酸化,从而引发CK1引起的Ser497磷酸化。这些磷酸化事件促进SRPK2核易位和SR蛋白的磷酸化。全基因组转录组分析表明,脂质生物合成酶是mTORC1-SRPK2信号转导的下游目标之一。从机理上讲,SRPK2促进SR蛋白与U1-70K的结合,从而诱导成脂前体mRNA的剪接。该信号通路的抑制导致内含子保留脂肪生成基因,从而触发无意义介导的mRNA衰变。SRPK2钝性从头合成脂质的遗传或药理抑制作用,从而抑制细胞生长。因此,这些结果揭示了mTORC1-SRPK2信号传导在脂质代谢的转录后调控中的新型作用,并证明SRPK2是mTORC1驱动的代谢性疾病的潜在治疗靶标。





























 京公网安备 11010802027423号
京公网安备 11010802027423号