当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rh(III)-catalyzed Redox-Neutral Unsymmetrical C-H Alkylation and Amidation Reactions of N-Phenoxyacetamides
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-26 , DOI: 10.1021/jacs.7b10349 Yunxiang Wu 1 , Zhaoqiang Chen , Yaxi Yang 1 , Weiliang Zhu , Bing Zhou 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2017-12-26 , DOI: 10.1021/jacs.7b10349 Yunxiang Wu 1 , Zhaoqiang Chen , Yaxi Yang 1 , Weiliang Zhu , Bing Zhou 1
Affiliation
|
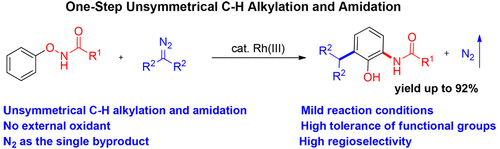
A Rh(III)-catalyzed unsymmetrical C-H alkylation and amidation of N-phenoxyacetamides with diazo compounds has been developed under mild and redox-neutral conditions, producing dinitrogen as the only byproduct. The reaction represents the first example of one-step, unsymmetrical difunctionalization of two ortho-C-H bonds. Experimental and computational studies support that N-phenoxyacetamides most likely undergo an initial ortho-C-H alkylation with diazo compounds via a Rh(III)-catalyzed C-H activation, and the resulting Rh(III) intermediate subsequently undergoes an intramolecular oxidative addition into the O-N bond to form a Rh(V) nitrenoid species that is protonated and further directed toward electrophilic addition to the second ortho position of the phenyl ring. This work might provide a new direction for unsymmetrical C-H difunctionalization reactions in an efficient manner.
中文翻译:

Rh(III)-催化氧化还原-中性不对称CH烷基化和N-苯氧基乙酰胺的酰胺化反应
已经在温和和氧化还原中性条件下开发了 Rh(III) 催化的 N-苯氧基乙酰胺与重氮化合物的不对称 CH 烷基化和酰胺化,产生二氮作为唯一的副产物。该反应代表了两个邻 CH 键的一步不对称双官能化的第一个例子。实验和计算研究支持 N-苯氧基乙酰胺最有可能通过 Rh(III) 催化的 CH 活化与重氮化合物进行初始邻位 CH 烷基化,所得的 Rh(III) 中间体随后经历分子内氧化加成到 ON 键形成 Rh(V) 氮烯类物质,该物质被质子化并进一步指向苯环的第二个邻位的亲电加成。
更新日期:2017-12-26
中文翻译:

Rh(III)-催化氧化还原-中性不对称CH烷基化和N-苯氧基乙酰胺的酰胺化反应
已经在温和和氧化还原中性条件下开发了 Rh(III) 催化的 N-苯氧基乙酰胺与重氮化合物的不对称 CH 烷基化和酰胺化,产生二氮作为唯一的副产物。该反应代表了两个邻 CH 键的一步不对称双官能化的第一个例子。实验和计算研究支持 N-苯氧基乙酰胺最有可能通过 Rh(III) 催化的 CH 活化与重氮化合物进行初始邻位 CH 烷基化,所得的 Rh(III) 中间体随后经历分子内氧化加成到 ON 键形成 Rh(V) 氮烯类物质,该物质被质子化并进一步指向苯环的第二个邻位的亲电加成。






























 京公网安备 11010802027423号
京公网安备 11010802027423号