当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spiropiperidine Sultam and Lactam Templates: Diastereoselective Overman Rearrangement and Metathesis followed by NH Arylation
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.joc.7b02096 Luis A. Martinez-Alsina 1 , John C. Murray 1 , Leanne M. Buzon 1 , Mark W. Bundesmann 1 , Joseph M. Young 2 , Brian T. O’Neill 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.joc.7b02096 Luis A. Martinez-Alsina 1 , John C. Murray 1 , Leanne M. Buzon 1 , Mark W. Bundesmann 1 , Joseph M. Young 2 , Brian T. O’Neill 1
Affiliation
|
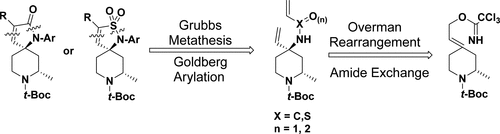
We report the diastereoselective synthesis of novel spiropiperidine templates for use in SAR studies of β-secretase (BACE) inhibitors and also as versatile ligands for other receptor types. The overall synthetic approach stems from chiral starting material benzyl (S)-2-methyl-4-oxopiperidine-1-carboxylate and employs an Overman rearrangement to control the stereochemistry at the quaternary center. This process is followed by a Grubbs metathesis to close a five-membered “top” ring to form an α,β-unsaturated lactam or an α,β-unsaturated sultam. We also demonstrate that this chemistry can accommodate additional substituents on the lactam/sultam ring and allows late stage sequential functionalization of the amine and amide nitrogens to rapidly produce diverse analogues.
中文翻译:

Spiropiperidine Sultam和Lactam模板:非对映选择性超人重排和复分解,然后进行NH酰化
我们报告了新型螺哌啶模板的非对映选择性合成,可用于β-分泌酶(BACE)抑制剂的SAR研究,也可作为其他受体类型的通用配体。整个合成方法均来自手性原料苄基(S)-2-甲基-4-氧杂哌啶-1-甲酸苄酯,并采用Overman重排控制四级中心的立体化学。在该过程之后,进行了格鲁布斯复分解,以封闭五元“顶”环,形成一个α,β-不饱和内酰胺或一个α,β-不饱和内酰胺。我们还证明了这种化学方法可以容纳内酰胺/舒马酰胺环上的其他取代基,并允许胺和酰胺氮的后期顺序功能化,以快速产生各种类似物。
更新日期:2017-11-16
中文翻译:

Spiropiperidine Sultam和Lactam模板:非对映选择性超人重排和复分解,然后进行NH酰化
我们报告了新型螺哌啶模板的非对映选择性合成,可用于β-分泌酶(BACE)抑制剂的SAR研究,也可作为其他受体类型的通用配体。整个合成方法均来自手性原料苄基(S)-2-甲基-4-氧杂哌啶-1-甲酸苄酯,并采用Overman重排控制四级中心的立体化学。在该过程之后,进行了格鲁布斯复分解,以封闭五元“顶”环,形成一个α,β-不饱和内酰胺或一个α,β-不饱和内酰胺。我们还证明了这种化学方法可以容纳内酰胺/舒马酰胺环上的其他取代基,并允许胺和酰胺氮的后期顺序功能化,以快速产生各种类似物。































 京公网安备 11010802027423号
京公网安备 11010802027423号