Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Surface Modification of Cisplatin-Complexed Gold Nanoparticles and Its Influence on Colloidal Stability, Drug Loading, and Drug Release
Langmuir ( IF 3.7 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acs.langmuir.7b02354 Jiaojie Tan,Tae Joon Cho,De-Hao Tsai,Jingyu Liu,John M. Pettibone,Rian You,Vincent A. Hackley,Michael R. Zachariah
Langmuir ( IF 3.7 ) Pub Date : 2017-12-18 00:00:00 , DOI: 10.1021/acs.langmuir.7b02354 Jiaojie Tan,Tae Joon Cho,De-Hao Tsai,Jingyu Liu,John M. Pettibone,Rian You,Vincent A. Hackley,Michael R. Zachariah
|
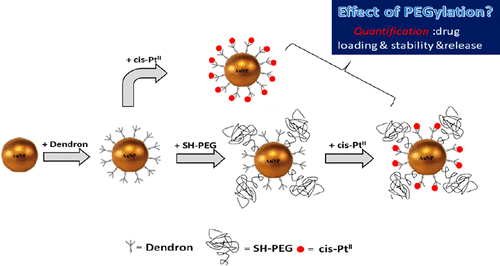
Cisplatin-complexed gold nanoparticles (PtII–AuNP) provide a promising strategy for chemo-radiation-based anticancer drugs. Effective design of such platforms necessitates reliable assessment of surface engineering on a quantitative basis and its influence on drug payload, stability, and release. In this paper, poly(ethylene glycol) (PEG)-stabilized PtII–AuNP was synthesized as a model antitumor drug platform, where PtII is attached via a carboxyl-terminated dendron ligand. Surface modification by PEG and its influence on drug loading, colloidal stability, and drug release were assessed. Complexation with PtII significantly degrades colloidal stability of the conjugate; however, PEGylation provides substantial improvement of stability in conjunction with an insignificant trade-off in drug loading capacity compared with the non-PEGylated control (<20% decrease in loading capacity). In this context, the effect of varying PEG concentration and molar mass was investigated. On a quantitative basis, the extent of PEGylation was characterized and its influence on dispersion stability and drug load was examined using electrospray differential mobility analysis (ES-DMA) hyphenated with inductively coupled plasma mass spectrometry (ICP-MS) and compared with attenuated total reflectance–FTIR. Using ES-DMA-ICP-MS, AuNP conjugates were size-classified based on their electrical mobility, while PtII loading was simultaneously quantified by determination of Pt mass. Colloidal stability was quantitatively evaluated in biologically relevant media. Finally, the pH-dependent PtII release performance was evaluated. We observed 9% and 16% PtII release at drug loadings of 0.5 and 1.9 PtII/nm2, respectively. The relative molar mass of PEG had no significant influence on PtII uptake or release performance, while PEGylation substantially improved the colloidal stability of the conjugate. Notably, the PtII release over 10 days (examined at 0.5 PtII/nm2 drug loading) remained constant for non-PEGylated, 1K-PEGylated, and 5K-PEGylated conjugates.
中文翻译:

顺铂复合金纳米粒子的表面修饰及其对胶体稳定性,载药量和药物释放的影响
顺铂复合金纳米颗粒(Pt II –AuNP)为基于化学辐射的抗癌药物提供了一种有前途的策略。这种平台的有效设计需要对表面工程进行定量的可靠评估,以及其对药物有效载荷,稳定性和释放的影响。在本文中,合成了聚乙二醇(PEG)稳定的Pt II –AuNP作为模型抗肿瘤药物平台,其中Pt II通过羧基末端的树枝状配体连接。评估了PEG的表面修饰及其对载药量,胶体稳定性和药物释放的影响。与Pt II络合显着降低缀合物的胶体稳定性;然而,与非聚乙二醇化的对照相比,聚乙二醇化可显着提高稳定性,同时药物载量也可以忽略不计(载量下降<20%)。在这种情况下,研究了改变PEG浓度和摩尔质量的影响。在定量基础上,表征了PEG化的程度,并使用电喷雾差分迁移率分析(ES-DMA)和电感耦合等离子体质谱(ICP-MS)进行了联用,研究了PEG化程度对分散稳定性和载药量的影响,并将其与衰减的全反射率进行了比较–FTIR。使用ES-DMA-ICP-MS,AuNP共轭物基于其电迁移率进行了大小分类,而Pt II通过测定Pt质量同时定量负载。在生物学相关介质中定量评估胶体稳定性。最后,评估了pH依赖性Pt II释放性能。我们观察到分别在0.5和1.9 Pt II / nm 2的载药量下释放了9%和16%的Pt II。PEG的相对摩尔质量对Pt II的摄取或释放性能没有显着影响,而PEG化大大改善了缀合物的胶体稳定性。值得注意的是,Pt II释放了10天(以0.5 Pt II / nm 2检查 对于非聚乙二醇化,1K聚乙二醇化和5K聚乙二醇化的缀合物,药物负载量保持恒定。
更新日期:2017-12-18
中文翻译:

顺铂复合金纳米粒子的表面修饰及其对胶体稳定性,载药量和药物释放的影响
顺铂复合金纳米颗粒(Pt II –AuNP)为基于化学辐射的抗癌药物提供了一种有前途的策略。这种平台的有效设计需要对表面工程进行定量的可靠评估,以及其对药物有效载荷,稳定性和释放的影响。在本文中,合成了聚乙二醇(PEG)稳定的Pt II –AuNP作为模型抗肿瘤药物平台,其中Pt II通过羧基末端的树枝状配体连接。评估了PEG的表面修饰及其对载药量,胶体稳定性和药物释放的影响。与Pt II络合显着降低缀合物的胶体稳定性;然而,与非聚乙二醇化的对照相比,聚乙二醇化可显着提高稳定性,同时药物载量也可以忽略不计(载量下降<20%)。在这种情况下,研究了改变PEG浓度和摩尔质量的影响。在定量基础上,表征了PEG化的程度,并使用电喷雾差分迁移率分析(ES-DMA)和电感耦合等离子体质谱(ICP-MS)进行了联用,研究了PEG化程度对分散稳定性和载药量的影响,并将其与衰减的全反射率进行了比较–FTIR。使用ES-DMA-ICP-MS,AuNP共轭物基于其电迁移率进行了大小分类,而Pt II通过测定Pt质量同时定量负载。在生物学相关介质中定量评估胶体稳定性。最后,评估了pH依赖性Pt II释放性能。我们观察到分别在0.5和1.9 Pt II / nm 2的载药量下释放了9%和16%的Pt II。PEG的相对摩尔质量对Pt II的摄取或释放性能没有显着影响,而PEG化大大改善了缀合物的胶体稳定性。值得注意的是,Pt II释放了10天(以0.5 Pt II / nm 2检查 对于非聚乙二醇化,1K聚乙二醇化和5K聚乙二醇化的缀合物,药物负载量保持恒定。































 京公网安备 11010802027423号
京公网安备 11010802027423号