当前位置:
X-MOL 学术
›
Ind. Eng. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solubility of Monomers for Chain Polymerization in Ionic Liquids Predicted by the Conductor-Like Screening Model for Real Solvents
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.iecr.7b04235 Xiaoqian Zhang 1, 2, 3 , Wenli Guo 1, 3 , Yibo Wu 1 , Wei Li 1 , Shuxin Li 1 , Yuwei Shang 1 , Jinghan Zhang 1, 3
Industrial & Engineering Chemistry Research ( IF 3.8 ) Pub Date : 2017-12-04 00:00:00 , DOI: 10.1021/acs.iecr.7b04235 Xiaoqian Zhang 1, 2, 3 , Wenli Guo 1, 3 , Yibo Wu 1 , Wei Li 1 , Shuxin Li 1 , Yuwei Shang 1 , Jinghan Zhang 1, 3
Affiliation
|
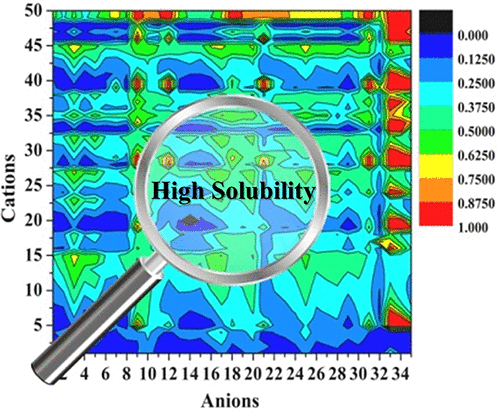
Ionic liquids have been extensively investigated as clean solvents for chain polymerization reactions, such as radical polymerization, anionic polymerization, and cationic polymerization. However, the low solubilities of the monomers in ionic liquids often impede the efficiency of these reactions. In this study, the solubilities of two typical vinyl monomers, namely, p-methylstyrene (p-MeSt) and isobutyl vinyl ether (IBVE) were studied in 1750 possible ionic liquids (combinations of 50 cations with 35 anions) by the conductor-like screening model for real solvents. The effects of the cation structures, anion structures, and ion chain length on the solubility were systemically studied. The interaction energies and σ profiles were also employed to explain major factors affecting solubility. The results revealed that a larger size of the nonpolar region of the cation or anion results in higher solubilities for p-MeSt and IBVE, as does a longer alkyl chain in cation or anion. In this study, a theoretical method for selecting ionic liquids for chain polymerization was established.
中文翻译:

实际溶剂中类似导体的筛选模型预测离子液体中链聚合单体的溶解度
离子液体作为链聚合反应的清洁溶剂已被广泛研究,例如自由基聚合,阴离子聚合和阳离子聚合。但是,单体在离子液体中的低溶解度通常会阻碍这些反应的效率。在这项研究中,两种典型的乙烯基单体中的溶解度,即,p甲基苯乙烯(p-MeSt)和异丁基乙烯基醚(IBVE)在1750种可能的离子液体(50个阳离子与35个阴离子的组合)中通过针对真实溶剂的类似导体的筛选模型进行了研究。系统研究了阳离子结构,阴离子结构和离子链长度对溶解度的影响。相互作用能和σ分布也被用来解释影响溶解度的主要因素。结果表明,阳离子或阴离子的非极性区域的较大尺寸导致p -MeSt和IBVE的溶解度较高,阳离子或阴离子中的较长烷基链也是如此。在这项研究中,建立了一种选择用于链聚合的离子液体的理论方法。
更新日期:2017-12-04
中文翻译:

实际溶剂中类似导体的筛选模型预测离子液体中链聚合单体的溶解度
离子液体作为链聚合反应的清洁溶剂已被广泛研究,例如自由基聚合,阴离子聚合和阳离子聚合。但是,单体在离子液体中的低溶解度通常会阻碍这些反应的效率。在这项研究中,两种典型的乙烯基单体中的溶解度,即,p甲基苯乙烯(p-MeSt)和异丁基乙烯基醚(IBVE)在1750种可能的离子液体(50个阳离子与35个阴离子的组合)中通过针对真实溶剂的类似导体的筛选模型进行了研究。系统研究了阳离子结构,阴离子结构和离子链长度对溶解度的影响。相互作用能和σ分布也被用来解释影响溶解度的主要因素。结果表明,阳离子或阴离子的非极性区域的较大尺寸导致p -MeSt和IBVE的溶解度较高,阳离子或阴离子中的较长烷基链也是如此。在这项研究中,建立了一种选择用于链聚合的离子液体的理论方法。

































 京公网安备 11010802027423号
京公网安备 11010802027423号