Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Clustered versus Uniform Display of GALA-Peptides on Carrier Nanoparticles: Enhancing the Permeation of Noncharged Fluid Lipid Membranes
Langmuir ( IF 3.7 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.langmuir.7b03706 Trevan Locke 1 , Stavroula Sofou 1
Langmuir ( IF 3.7 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.langmuir.7b03706 Trevan Locke 1 , Stavroula Sofou 1
Affiliation
|
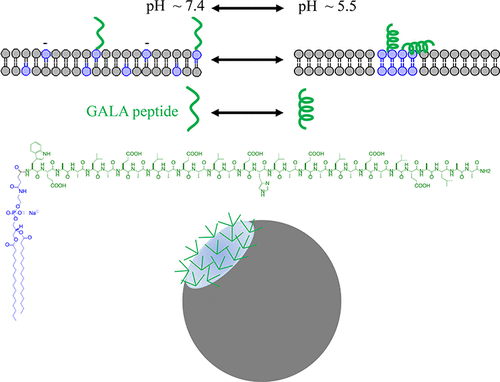
GALA-peptide is a random coil in neutral pH; in acidic pH, it becomes an amphipathic α-helix that aggregates in solution, possibly via its hydrophobic facet that runs along the helix’s long axis. In the presence of fluid lipid membranes, the GALA-helix exhibits membrane-active properties that originate from the same hydrophobic facet; these properties make GALA a candidate for inclusion in drug delivery systems requiring permeation of the endosomal membrane to enable drug escape into the cytoplasm. Previous work has shown that the uniform functionalization of carrier nanoparticles with GALA-peptides improved their membrane activity and enhanced the endosomal escape of delivered therapeutics. The present study aims to evaluate the potential role of altering membrane activity via cluster-displayed GALA-peptides (for higher local valency) on the surface of carrier nanoparticles. The presentation of GALA-peptides on carrier nanoparticles was also designed to be pH-dependent. The peptide display on the surface of the carrier nanoparticles was uniform in neutral pH; in the acidic endosomal pH, the surface of nanocarriers formed domains (patches) with high local densities of GALA-peptides. The interactions between GALA-functionalized carrier nanoparticles and target lipid vesicles, utilized as endosome membrane surrogates that were used to primarily capture the fluid nature of these membranes, were studied as a function of pH. At endosomal pH values, ranging from 5.5 to 5.0, the greatest permeability of target membranes was induced by nanocarriers with clustered rather than uniformly displayed GALA. This enhancing effect had an optimum; at even more acidic pH values, too close an approximation of GALA-peptides residing within the same patches resulted in preferential intrapatch peptide interactions rather than interactions with the apposing target lipid membranes. This behavior could have the same physicochemical origin as the aforementioned GALA-peptide aggregation, observed in solution with decreasing pH at increasing peptide concentrations. The findings of this study support the potential of utilizing the clustered display of GALA-peptides on carrier nanoparticles to increase the permeation of fluid membranes used herein to capture this critical physical property of endosomal membranes and therefore to ultimately improve the endosomal escape of delivered therapeutics, enhancing therapeutic efficacy.
中文翻译:

在载体纳米颗粒上的聚类与统一显示GALA肽:增强不带电荷的流体脂质膜的渗透。
GALA肽是中性pH值的无规卷曲;在酸性pH值下,它可能变成两亲性α-螺旋,并可能通过其沿着螺旋长轴延伸的疏水面在溶液中聚集。在存在液体脂质膜的情况下,GALA-螺旋表现出源自相同疏水性小面的膜活性特性。这些特性使GALA成为包含在需要通过内体膜渗透以使药物逸出进入细胞质的药物输送系统中的候选药物。先前的工作表明,带有GALA肽的载体纳米颗粒的均匀功能性改善了其膜活性,并增强了所递送治疗剂的内体逸出。本研究旨在评估通过在载体纳米颗粒表面上簇展示的GALA肽(用于更高的化合价)改变膜活性的潜在作用。GALA肽在载体纳米颗粒上的呈递也被设计为pH依赖性的。载体纳米颗粒表面上的肽展示在中性pH下是均匀的;在酸性内体pH中,纳米载体的表面形成了具有高局部密度GALA肽的结构域(斑块)。研究了GALA功能化的载体纳米颗粒与目标脂质囊泡之间的相互作用,这些脂质体被用作内吞膜替代物,主要用于捕获这些膜的流体性质,并随pH的变化而变化。内体pH值介于5.5至5.0之间时,具有簇状而不是均匀展示的GALA的纳米载体诱导了最大的目标膜通透性。这种增强效果具有最佳效果。在甚至更高的酸性pH值下,驻留在相同贴片中的GALA肽的近似值太近,会导致优先的Intrapatch肽相互作用,而不是与相应的目标脂质膜相互作用。在溶液中随着肽浓度的增加而pH降低时,这种行为可能具有与上述GALA肽聚集相同的物理化学起源。
更新日期:2017-11-14
中文翻译:

在载体纳米颗粒上的聚类与统一显示GALA肽:增强不带电荷的流体脂质膜的渗透。
GALA肽是中性pH值的无规卷曲;在酸性pH值下,它可能变成两亲性α-螺旋,并可能通过其沿着螺旋长轴延伸的疏水面在溶液中聚集。在存在液体脂质膜的情况下,GALA-螺旋表现出源自相同疏水性小面的膜活性特性。这些特性使GALA成为包含在需要通过内体膜渗透以使药物逸出进入细胞质的药物输送系统中的候选药物。先前的工作表明,带有GALA肽的载体纳米颗粒的均匀功能性改善了其膜活性,并增强了所递送治疗剂的内体逸出。本研究旨在评估通过在载体纳米颗粒表面上簇展示的GALA肽(用于更高的化合价)改变膜活性的潜在作用。GALA肽在载体纳米颗粒上的呈递也被设计为pH依赖性的。载体纳米颗粒表面上的肽展示在中性pH下是均匀的;在酸性内体pH中,纳米载体的表面形成了具有高局部密度GALA肽的结构域(斑块)。研究了GALA功能化的载体纳米颗粒与目标脂质囊泡之间的相互作用,这些脂质体被用作内吞膜替代物,主要用于捕获这些膜的流体性质,并随pH的变化而变化。内体pH值介于5.5至5.0之间时,具有簇状而不是均匀展示的GALA的纳米载体诱导了最大的目标膜通透性。这种增强效果具有最佳效果。在甚至更高的酸性pH值下,驻留在相同贴片中的GALA肽的近似值太近,会导致优先的Intrapatch肽相互作用,而不是与相应的目标脂质膜相互作用。在溶液中随着肽浓度的增加而pH降低时,这种行为可能具有与上述GALA肽聚集相同的物理化学起源。































 京公网安备 11010802027423号
京公网安备 11010802027423号