当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Speciation of CuCl and CuCl2 Thiol-Amine Solutions and Characterization of Resulting Films: Implications for Semiconductor Device Fabrication
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01359 Priya Murria 1 , Caleb K. Miskin 2 , Robert Boyne 2 , Laurance T. Cain 1 , Ravikiran Yerabolu 1 , Ruihong Zhang 3 , Evan C. Wegener 2 , Jeffrey T. Miller 2 , Hilkka I. Kenttämaa 1 , Rakesh Agrawal 2
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.inorgchem.7b01359 Priya Murria 1 , Caleb K. Miskin 2 , Robert Boyne 2 , Laurance T. Cain 1 , Ravikiran Yerabolu 1 , Ruihong Zhang 3 , Evan C. Wegener 2 , Jeffrey T. Miller 2 , Hilkka I. Kenttämaa 1 , Rakesh Agrawal 2
Affiliation
|
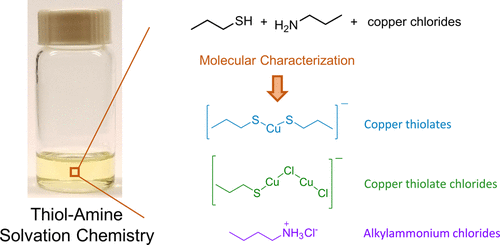
Thiol-amine mixtures are an attractive medium for the solution processing of semiconducting thin films because of their remarkable ability to dissolve a variety of metals, metal chalcogenides, metal salts, and chalcogens. However, very little is known about their dissolution chemistry. Electrospray ionization high-resolution tandem mass spectrometry and X-ray absorption spectroscopy were employed to identify the species formed upon dissolution of CuCl and CuCl2 in 1-propanethiol and n-butylamine. Copper was found to be present exclusively in the 1+ oxidation state for both solutions. The copper complexes detected include copper chlorides, copper thiolates, and copper thiolate chlorides. No complexes of copper with amines were observed. Additionally, alkylammonium ions and alkylammonium chloride adducts were observed. These findings suggest that the dissolution is initiated by proton transfer from the thiol to the amine, followed by coordination of the thiolate anions with copper cations. Interestingly, the mass and X-ray absorption spectra of the solutions of CuCl and CuCl2 in thiol-amine were essentially identical. However, dialkyl disulfides were identified by Raman spectroscopy as an oxidation product only for the copper(II) solution, wherein copper(II) had been reduced to copper(I). Analysis of several thiol-amine pairs suggested that the dissolution mechanism is quite general. Finally, analysis of thin films prepared from these solutions revealed persistent chlorine impurities, in agreement with previous studies. These impurities are explained by the mass spectrometric finding that chloride ligands are not completely displaced by thiolates upon dissolution. These results suggest that precursors other than chlorides will likely be preferred for the generation of high-efficiency copper chalcogenide films, despite the reasonable efficiencies that have been obtained for films generated from chloride precursors in the past.
中文翻译:

CuCl和CuCl 2硫胺溶液的形态和所得膜的表征:对半导体器件制造的启示
硫醇-胺混合物是半导体薄膜溶液加工的一种有吸引力的介质,因为它们具有溶解多种金属,金属硫属元素化物,金属盐和硫属元素素的出色能力。但是,对其溶解化学知之甚少。电喷雾电离高分辨率串联质谱法和X射线吸收光谱法用于鉴定将CuCl和CuCl 2溶解在1-丙硫醇和n中形成的物质-丁胺。对于这两种溶液,发现铜仅以1+氧化态存在。检测到的铜络合物包括氯化铜,硫醇铜和硫醇铜氯化物。没有观察到铜与胺的络合物。另外,观察到烷基铵离子和烷基铵氯化物加合物。这些发现表明,溶解是通过质子从硫醇转移到胺中引发的,然后硫醇盐阴离子与铜阳离子的配位而开始的。有趣的是,CuCl和CuCl 2溶液的质量和X射线吸收光谱在硫醇胺中基本相同。然而,二烷基二硫化物通过拉曼光谱法鉴定为仅对于铜(II)溶液的氧化产物,其中铜(II)已被还原为铜(I)。对几个硫醇-胺对的分析表明,其溶解机理是相当普遍的。最后,与以前的研究一致,从这些溶液制备的薄膜的分析显示出持久的氯杂质。这些杂质可以通过质谱分析来解释,即氯化物配体在溶解时不会被硫醇盐完全取代。这些结果表明,除氯化物以外的其他前体可能会更适合生成高效硫属元素铜薄膜,
更新日期:2017-11-13
中文翻译:

CuCl和CuCl 2硫胺溶液的形态和所得膜的表征:对半导体器件制造的启示
硫醇-胺混合物是半导体薄膜溶液加工的一种有吸引力的介质,因为它们具有溶解多种金属,金属硫属元素化物,金属盐和硫属元素素的出色能力。但是,对其溶解化学知之甚少。电喷雾电离高分辨率串联质谱法和X射线吸收光谱法用于鉴定将CuCl和CuCl 2溶解在1-丙硫醇和n中形成的物质-丁胺。对于这两种溶液,发现铜仅以1+氧化态存在。检测到的铜络合物包括氯化铜,硫醇铜和硫醇铜氯化物。没有观察到铜与胺的络合物。另外,观察到烷基铵离子和烷基铵氯化物加合物。这些发现表明,溶解是通过质子从硫醇转移到胺中引发的,然后硫醇盐阴离子与铜阳离子的配位而开始的。有趣的是,CuCl和CuCl 2溶液的质量和X射线吸收光谱在硫醇胺中基本相同。然而,二烷基二硫化物通过拉曼光谱法鉴定为仅对于铜(II)溶液的氧化产物,其中铜(II)已被还原为铜(I)。对几个硫醇-胺对的分析表明,其溶解机理是相当普遍的。最后,与以前的研究一致,从这些溶液制备的薄膜的分析显示出持久的氯杂质。这些杂质可以通过质谱分析来解释,即氯化物配体在溶解时不会被硫醇盐完全取代。这些结果表明,除氯化物以外的其他前体可能会更适合生成高效硫属元素铜薄膜,





















































 京公网安备 11010802027423号
京公网安备 11010802027423号