当前位置:
X-MOL 学术
›
Inorg. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Insertion of a Transient Tin Nitride into Carbon–Carbon and Boron–Carbon Bonds
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02413 Shuai Wang 1 , Lizhi Tao 1 , Troy A. Stich 1 , Marilyn M. Olmstead 1 , R. David Britt 1 , Philip P. Power
Inorganic Chemistry ( IF 4.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acs.inorgchem.7b02413 Shuai Wang 1 , Lizhi Tao 1 , Troy A. Stich 1 , Marilyn M. Olmstead 1 , R. David Britt 1 , Philip P. Power
Affiliation
|
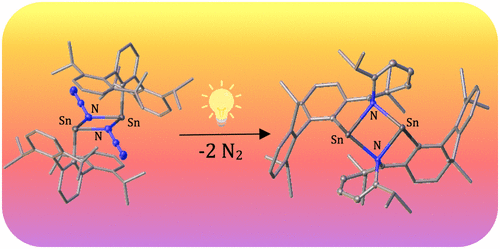
A simple exchange reaction between [AriPr4Sn(μ-Cl)]2 (1) and sodium azide afforded the doubly bridged Sn(II) azide, [AriPr4Sn(μ-N3)]2 (2) (AriPr4 = C6H3-2,6(C6H3-2,6-iPr2)2) in 85% yield. Photolysis of a diethyl ether solution of 2 for ca. 16 h yielded an azepinyl-substituted insertion product, [C6H3-2-(C6H3-2,6-iPr2)-6-(C6H3N-3,7-iPr2)Sn]2 (3). The reaction of the Lewis acid, B(C6F5)3 (BCF), or the Lewis base, pyridine, with 2 dissociates the dimer to afford the corresponding complexed monomeric Sn(II) azide, AriPr4SnN3BCF (4) in which BCF coordinates the α-nitrogen, or AriPr4Sn(pyridine)N3 (6) in which pyridine coordinates to the tin atom. Photolysis of 4 in diethyl ether for 12 h results in the insertion of the α-nitrogen of the azide group into one of the B–C bonds of the BCF acceptor to yield the tin(II) amide, AriPr4SnN(C6F5)B(C6F5)2 (5). In contrast, photolysis of 6 for over 36 h afforded no apparent reaction. A highly reactive Sn nitride intermediate, AriPr4Sn≡N, is proposed as part of the mechanistic pathway for the formation of 3 and 5 as a result of trapping the tin-centered radical isomers. This was effected by immediate freezing the samples of 2 or 4 after ca. 30 min of UV photolysis and recording their electron paramagnetic resonance spectra. These exhibited a rhombic g tensor of [g1, g2, g3] = [2.029, 1.978, 1.933]. This radical intermediate could be related to the valence isomers of the nitride [−SnIV≡N] intermediate, in isomeric equilibrium with the nitrene [−SnII–N] and nitridyl [−SnIII═N·] forms, but with the spin density on the nitrogen being quenched, possibly by the H atom abstraction to form an S = 1/2 species of formula −Sn·═N(H).
中文翻译:

将瞬态氮化锡插入碳-碳和硼-碳键中
之间[氩一个简单的交换反应IPR4锡(μ-Cl)的] 2(1叠氮化物)和钠得到双桥联的Sn(II)叠氮化物,[氩IPR4的Sn(μ-N 3)] 2(2)(氩IPR4 = C 6 H ^ 3 -2,6(C 6 H ^ 3 -2,6-我镨2)2)在85%的产率。2的二乙醚溶液的光解时间约为1分钟。16小时得到氮杂环丁烷基取代的插入产物,[C 6 H 3 -2-(C 6 H 3 -2,6-i Pr 2)-6-(C 6 H 3 N-3,7- i Pr 2)Sn] 2( 3)。Lewis酸B(C 6 F 5) 3(BCF)或Lewis碱吡啶与2的反应使二聚体解离,得到相应的络合单体叠氮化Sn(II)Ar iPr4 SnN 3 BCF( 4),其中BCF配位α-氮,或Ar iPr4 Sn(吡啶)N 3( 6),其中吡啶配位至锡原子。4的光解在乙醚中放置12小时会导致叠氮化物基团的α-氮插入BCF受体的B–C键之一,从而生成锡(II)酰胺Ar iPr4 SnN(C 6 F 5)B (C 6 F 5)2(5)。相比之下,6的光解作用超过36小时没有产生明显的反应。作为捕获锡中心自由基异构体的结果,提出了一种高反应性的氮化锡中间体Ar iPr4Sn≡N,作为形成3和5的机理途径的一部分。这是通过立即冷冻2或4个样品来实现的后约。30分钟的紫外线光解,并记录其电子顺磁共振光谱。这些表现出[ g 1,g 2,g 3 ]的菱形g张量= [2.029,1.978,1.933]。这种自由基中间体可能与氮化物[-Sn IV -N]中间体的价态异构体有关,处于与亚硝基[-Sn II -N]和亚硝基[-Sn III -N]形式的异构体平衡状态,但与氮上的自旋密度被淬灭,可能是通过H原子抽象形成S = 1/2式-Sn·═N(H)的。
更新日期:2017-11-13
中文翻译:

将瞬态氮化锡插入碳-碳和硼-碳键中
之间[氩一个简单的交换反应IPR4锡(μ-Cl)的] 2(1叠氮化物)和钠得到双桥联的Sn(II)叠氮化物,[氩IPR4的Sn(μ-N 3)] 2(2)(氩IPR4 = C 6 H ^ 3 -2,6(C 6 H ^ 3 -2,6-我镨2)2)在85%的产率。2的二乙醚溶液的光解时间约为1分钟。16小时得到氮杂环丁烷基取代的插入产物,[C 6 H 3 -2-(C 6 H 3 -2,6-i Pr 2)-6-(C 6 H 3 N-3,7- i Pr 2)Sn] 2( 3)。Lewis酸B(C 6 F 5) 3(BCF)或Lewis碱吡啶与2的反应使二聚体解离,得到相应的络合单体叠氮化Sn(II)Ar iPr4 SnN 3 BCF( 4),其中BCF配位α-氮,或Ar iPr4 Sn(吡啶)N 3( 6),其中吡啶配位至锡原子。4的光解在乙醚中放置12小时会导致叠氮化物基团的α-氮插入BCF受体的B–C键之一,从而生成锡(II)酰胺Ar iPr4 SnN(C 6 F 5)B (C 6 F 5)2(5)。相比之下,6的光解作用超过36小时没有产生明显的反应。作为捕获锡中心自由基异构体的结果,提出了一种高反应性的氮化锡中间体Ar iPr4Sn≡N,作为形成3和5的机理途径的一部分。这是通过立即冷冻2或4个样品来实现的后约。30分钟的紫外线光解,并记录其电子顺磁共振光谱。这些表现出[ g 1,g 2,g 3 ]的菱形g张量= [2.029,1.978,1.933]。这种自由基中间体可能与氮化物[-Sn IV -N]中间体的价态异构体有关,处于与亚硝基[-Sn II -N]和亚硝基[-Sn III -N]形式的异构体平衡状态,但与氮上的自旋密度被淬灭,可能是通过H原子抽象形成S = 1/2式-Sn·═N(H)的。

































 京公网安备 11010802027423号
京公网安备 11010802027423号