当前位置:
X-MOL 学术
›
ACS Appl. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Series of Lithium Pyridyl Phenolate Complexes with a Pendant Pyridyl Group for Electron-Injection Layers in Organic Light-Emitting Devices
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acsami.7b13550 Satoru Ohisa , Taichiro Karasawa , Yuichiro Watanabe , Tatsuya Ohsawa , Yong-Jin Pu , Tomoyuki Koganezawa 1 , Hisahiro Sasabe , Junji Kido
ACS Applied Materials & Interfaces ( IF 8.3 ) Pub Date : 2017-11-13 00:00:00 , DOI: 10.1021/acsami.7b13550 Satoru Ohisa , Taichiro Karasawa , Yuichiro Watanabe , Tatsuya Ohsawa , Yong-Jin Pu , Tomoyuki Koganezawa 1 , Hisahiro Sasabe , Junji Kido
Affiliation
|
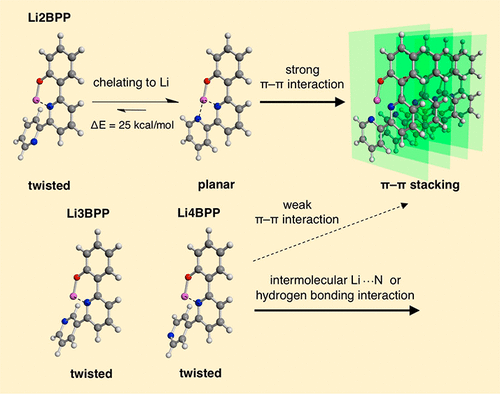
We report a new series of lithium pyridyl phenolate complexes with a pendant pyridyl group, Li2BPP, Li3BPP, and Li4BPP, in which the pendant pyridines are substituted at the 2-, 3-, and 4-positions, respectively. The most important difference between these complexes is their molecular planarity; Li3BPP and Li4BPP adopt twisted bipyridine structures, whereas Li2BPP adopts a planar structure owing to the steric hindrance and chelating effect of bipyridine on the Li core. The planar structure leads to crystallization through π–π stacking interactions, and the small differences in the molecular structures of the pendant pyridine rings cause drastic differences in the physical properties of thin solid films of these complexes. We applied these complexes as electron-injection layers (EILs) in Ir(ppy)3-based organic light-emitting devices. When thin EILs were used, Li3BPP and Li4BPP afforded lower driving voltages than Li2BPP; the order of the driving voltages followed the order of their electron affinity values. Moreover, the dependence of driving voltage on the EIL thickness was investigated for each complex. Among the three LiBPP derivatives, Li2BPP-based devices showed almost negligible EIL thickness dependence, which may be attributable to the high crystallinity of Li2BPP. All LiBPP-based devices also showed higher stability than conventional 8-quinolinolato lithium-based devices.
中文翻译:

有机发光器件中电子注入层的一系列带有悬垂吡啶基的吡啶甲酸锂吡啶酯
我们报告了一系列新的吡啶基锂吡啶酚锂复合物,其中有吡啶基侧基Li2BPP,Li3BPP和Li4BPP,其中吡啶侧基分别在2、3和4位被取代。这些配合物之间最重要的区别是它们的分子平面性。Li3BPP和Li4BPP采用扭曲的联吡啶结构,而Li2BPP由于联吡啶对Li核的位阻和螯合作用而采用平面结构。平面结构通过π-π堆积相互作用导致结晶,而吡啶环侧链分子结构的微小差异导致这些配合物的固体薄膜的物理性质发生巨大差异。我们将这些络合物用作Ir(ppy)3中的电子注入层(EIL)的有机发光器件。当使用薄EIL时,Li3BPP和Li4BPP提供的驱动电压低于Li2BPP。驱动电压的顺序遵循其电子亲和力值的顺序。此外,研究了每种复合物的驱动电压对EIL厚度的依赖性。在这三种LiBPP衍生物中,基于Li2BPP的器件显示出几乎可以忽略的EIL厚度依赖性,这可能归因于Li2BPP的高结晶度。所有基于LiBPP的设备还显示出比传统的8喹啉基对锂基设备更高的稳定性。
更新日期:2017-11-14
中文翻译:

有机发光器件中电子注入层的一系列带有悬垂吡啶基的吡啶甲酸锂吡啶酯
我们报告了一系列新的吡啶基锂吡啶酚锂复合物,其中有吡啶基侧基Li2BPP,Li3BPP和Li4BPP,其中吡啶侧基分别在2、3和4位被取代。这些配合物之间最重要的区别是它们的分子平面性。Li3BPP和Li4BPP采用扭曲的联吡啶结构,而Li2BPP由于联吡啶对Li核的位阻和螯合作用而采用平面结构。平面结构通过π-π堆积相互作用导致结晶,而吡啶环侧链分子结构的微小差异导致这些配合物的固体薄膜的物理性质发生巨大差异。我们将这些络合物用作Ir(ppy)3中的电子注入层(EIL)的有机发光器件。当使用薄EIL时,Li3BPP和Li4BPP提供的驱动电压低于Li2BPP。驱动电压的顺序遵循其电子亲和力值的顺序。此外,研究了每种复合物的驱动电压对EIL厚度的依赖性。在这三种LiBPP衍生物中,基于Li2BPP的器件显示出几乎可以忽略的EIL厚度依赖性,这可能归因于Li2BPP的高结晶度。所有基于LiBPP的设备还显示出比传统的8喹啉基对锂基设备更高的稳定性。































 京公网安备 11010802027423号
京公网安备 11010802027423号