当前位置:
X-MOL 学术
›
Catal. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The controlled catalytic oxidation of furfural to furoic acid using AuPd/Mg(OH)2
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-07-14 00:00:00 , DOI: 10.1039/c7cy01025g
Mark Douthwaite 1, 2, 3, 4 , Xiaoyang Huang 1, 2, 3, 4 , Sarwat Iqbal 1, 2, 3, 4 , Peter J. Miedziak 1, 2, 3, 4 , Gemma L. Brett 1, 2, 3, 4 , Simon A. Kondrat 1, 2, 3, 4 , Jennifer K. Edwards 1, 2, 3, 4 , Meenakshisundaram Sankar 1, 2, 3, 4 , David W. Knight 1, 2, 3, 4 , Donald Bethell 1, 2, 3, 4 , Graham J. Hutchings 1, 2, 3, 4
Catalysis Science & Technology ( IF 4.4 ) Pub Date : 2017-07-14 00:00:00 , DOI: 10.1039/c7cy01025g
Mark Douthwaite 1, 2, 3, 4 , Xiaoyang Huang 1, 2, 3, 4 , Sarwat Iqbal 1, 2, 3, 4 , Peter J. Miedziak 1, 2, 3, 4 , Gemma L. Brett 1, 2, 3, 4 , Simon A. Kondrat 1, 2, 3, 4 , Jennifer K. Edwards 1, 2, 3, 4 , Meenakshisundaram Sankar 1, 2, 3, 4 , David W. Knight 1, 2, 3, 4 , Donald Bethell 1, 2, 3, 4 , Graham J. Hutchings 1, 2, 3, 4
Affiliation
|
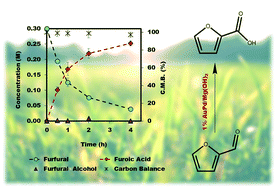
The emphasis of modern chemistry is to satisfy the needs of consumers by using methods that are sustainable and economical. Using a 1% AuPd/Mg(OH)2 catalyst in the presence of NaOH and under specific reaction conditions furfural; a platform chemical formed from lignocellulosic biomass, can be selectively oxidised to furoic acid, and the catalyst displays promising reusability for this reaction. The mechanism of this conversion is complex with multiple competing pathways possible. The experimental conditions and AuPd metal ratio can be fine-tuned to provide enhanced control of the reaction selectivity. Activation energies were derived for the homogeneous Cannizzaro pathway and the catalytic oxidation of furfural using the initial rates methodology. This work highlights the potential of using a heterogeneous catalyst for the oxidation of furfural to furoic acid that has potential for commercial application.
中文翻译:

使用AuPd / Mg(OH)2将糠醛受控催化氧化为糠酸
现代化学的重点是通过使用可持续且经济的方法来满足消费者的需求。使用1%AuPd / Mg(OH)2在NaOH存在下和在特定反应条件下糠醛的催化剂;由木质纤维素生物质形成的平台化学品可以选择性地氧化为糠酸,并且该催化剂在该反应中显示出可重复使用的前景。这种转化的机制很复杂,可能存在多种竞争途径。可以对实验条件和AuPd金属比进行微调,以增强对反应选择性的控制。使用初始速率方法获得了均相Cannizzaro途径和糠醛催化氧化的活化能。这项工作突出了使用非均相催化剂将糠醛氧化为糠酸的潜力,这具有商业应用潜力。
更新日期:2017-11-14
中文翻译:

使用AuPd / Mg(OH)2将糠醛受控催化氧化为糠酸
现代化学的重点是通过使用可持续且经济的方法来满足消费者的需求。使用1%AuPd / Mg(OH)2在NaOH存在下和在特定反应条件下糠醛的催化剂;由木质纤维素生物质形成的平台化学品可以选择性地氧化为糠酸,并且该催化剂在该反应中显示出可重复使用的前景。这种转化的机制很复杂,可能存在多种竞争途径。可以对实验条件和AuPd金属比进行微调,以增强对反应选择性的控制。使用初始速率方法获得了均相Cannizzaro途径和糠醛催化氧化的活化能。这项工作突出了使用非均相催化剂将糠醛氧化为糠酸的潜力,这具有商业应用潜力。































 京公网安备 11010802027423号
京公网安备 11010802027423号