当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Stereoselective synthesis of highly branched chiral cyclobutane-cored triamines and their conjugation to Gd-DOTA
Tetrahedron ( IF 2.1 ) Pub Date : 2015-09-21 14:42:17
Jimena Ospina, Raquel Gutiérrez-Abad, Silvia Lope-Piedrafita, Ona Illa, Vicenç Branchadell, Rosa M. Ortuño
Tetrahedron ( IF 2.1 ) Pub Date : 2015-09-21 14:42:17
Jimena Ospina, Raquel Gutiérrez-Abad, Silvia Lope-Piedrafita, Ona Illa, Vicenç Branchadell, Rosa M. Ortuño
|
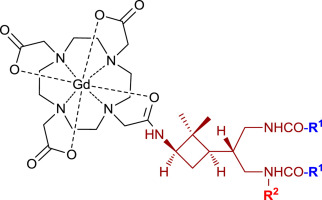
Several chiral cyclobutane-containing chemical platforms were synthesized in a stereoselective manner starting from (−)-verbenone as a suitable precursor. These compounds bear three orthogonally protected amine functions, two of them on side-chain and the other directly linked to the cyclobutane ring. Selective deprotection of the latter amine and subsequent coupling with the DOTA macrocycle followed by complexation with Gd(III) allowed the preparation of new GdDO3A monoamides whose potential ability as contrast agents (CAs) in magnetic resonance imaging (MRI) preliminary experiments was tested. Image contrast enhancement was shown to be dependent on the amine substitution and protecting groups (–NH–Cbz, –NH–CO–C6H4–p-NO2, –NH–Ac, and –NMe–Cbz), and in the case of acetamide the corresponding Gd complex manifested a better contrast enhancement than DOTAREM, a Gd-based commercial CA which was used as a reference. Computational studies suggest that water exchange rate in these complexes is associated to the ability of the amido-carbonyl groups in the cyclobutane side-chains to coordinate to Gd.
中文翻译:

高支化手性环丁烷-核三胺的立体选择性合成及其与Gd-DOTA的结合
以(-)-马来酮为合适的前体,以立体选择性的方式合成了几种含手性环丁烷的化学平台。这些化合物具有三个正交保护的胺官能团,其中两个在侧链上,另一个直接与环丁烷环相连。后一种胺的选择性脱保护并随后与DOTA大环偶联,然后与Gd(III)络合,从而可以制备新的GdDO3A单酰胺,其在磁共振成像(MRI)初步实验中作为造影剂(CAs)的潜在能力得到了测试。显示图像对比度增强取决于胺取代基和保护基团(–NH–Cbz,–NH–CO–C 6 H 4 –p-NO 2,-NH-Ac和-NMe-Cbz),并且在乙酰胺的情况下,相应的Gd络合物比DOTAREM(基于Gd的商业CA)作为参考具有更好的对比度增强。计算研究表明,这些配合物中的水交换速率与环丁烷侧链中酰胺基羰基与Gd配位的能力有关。
更新日期:2015-09-22
中文翻译:

高支化手性环丁烷-核三胺的立体选择性合成及其与Gd-DOTA的结合
以(-)-马来酮为合适的前体,以立体选择性的方式合成了几种含手性环丁烷的化学平台。这些化合物具有三个正交保护的胺官能团,其中两个在侧链上,另一个直接与环丁烷环相连。后一种胺的选择性脱保护并随后与DOTA大环偶联,然后与Gd(III)络合,从而可以制备新的GdDO3A单酰胺,其在磁共振成像(MRI)初步实验中作为造影剂(CAs)的潜在能力得到了测试。显示图像对比度增强取决于胺取代基和保护基团(–NH–Cbz,–NH–CO–C 6 H 4 –p-NO 2,-NH-Ac和-NMe-Cbz),并且在乙酰胺的情况下,相应的Gd络合物比DOTAREM(基于Gd的商业CA)作为参考具有更好的对比度增强。计算研究表明,这些配合物中的水交换速率与环丁烷侧链中酰胺基羰基与Gd配位的能力有关。

































 京公网安备 11010802027423号
京公网安备 11010802027423号