Bioorganic Chemistry ( IF 4.5 ) Pub Date : 2017-11-02 , DOI: 10.1016/j.bioorg.2017.10.021 Shahbaz Shamim , Khalid Mohammed Khan , Uzma Salar , Farman Ali , Muhammad Arif Lodhi , Muhammad Taha , Farman Ali Khan , Sajda Ashraf , Zaheer Ul-Haq , Muhammad Ali , Shahnaz Perveen
|
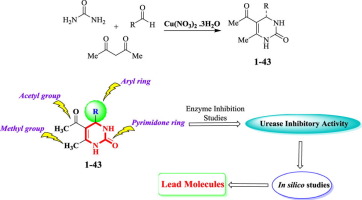
5-Acetyl-6-methyl-4-aryl-3,4-dihydropyrimidin-2(1H)-ones 1–43 were synthesized in a “one-pot” three component reaction and structurally characterized by various spectroscopic techniques such as 1H, 13C NMR, EI-MS, HREI-MS, and IR. All compounds were evaluated for their in vitro urease inhibitory activity. It is worth mentioning that except derivatives 1, 11, 12, and 14, all were found to be more potent than the standard thiourea (IC50 = 21.25 ± 0.15 µM) and showed their urease inhibitory potential in the range of IC50 = 3.70 ± 0.5–20.14 ± 0.1 µM. Structure-activity relationship (SAR) was rationalized by looking at the varying structural features of the molecules. However, molecular modeling study was performed to confirm the binding interactions of the molecules (ligand) with the active site of enzyme.
中文翻译:

5-乙酰基-6-甲基-4-芳基-3,4-二氢嘧啶-2(1 H)-ones:作为有效的脲酶抑制剂;合成,体外筛选和分子模型研究
5-乙酰基-6-甲基-4-芳基-3,4-二氢嘧啶-2(ħ) -酮1 - 43在“一锅”三个组分反应,合成,并通过各种光谱技术如结构表征1 1 H,13 C NMR,EI-MS,HREI-MS和IR。评价所有化合物的体外脲酶抑制活性。值得一提的是,除了衍生物1,11,12,和14,所有的被认为是比标准硫脲更有效(IC 50 = 21.25±0.15μM),并显示它们的脲酶抑制潜力在IC的范围50 = 3.70±0.5–20.14±0.1 µM。通过观察分子的不同结构特征来合理化结构-活性关系(SAR)。但是,进行了分子建模研究以确认分子(配体)与酶的活性位点的结合相互作用。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号