当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structural and Functional Characterization of a Hole–Hole Homodimer Variant in a “Knob-Into-Hole” Bispecific Antibody
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acs.analchem.7b03830 Hui-Min Zhang , Charlene Li , Ming Lei , Victor Lundin , Ho Young Lee , Milady Ninonuevo , Kevin Lin , Guanghui Han , Wendy Sandoval , Dongsheng Lei 1 , Gang Ren 1 , Jennifer Zhang , Hongbin Liu
Analytical Chemistry ( IF 6.7 ) Pub Date : 2017-12-01 00:00:00 , DOI: 10.1021/acs.analchem.7b03830 Hui-Min Zhang , Charlene Li , Ming Lei , Victor Lundin , Ho Young Lee , Milady Ninonuevo , Kevin Lin , Guanghui Han , Wendy Sandoval , Dongsheng Lei 1 , Gang Ren 1 , Jennifer Zhang , Hongbin Liu
Affiliation
|
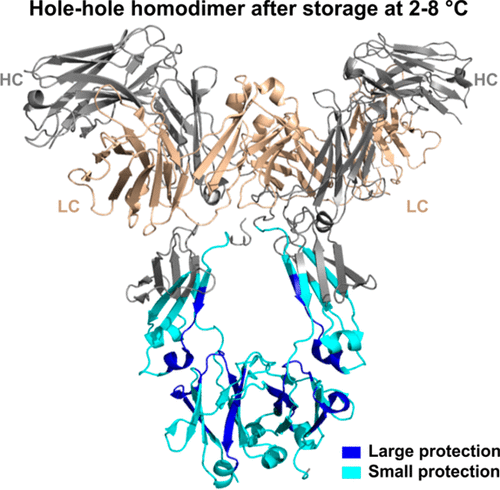
Bispecific antibodies have great potential to be the next-generation biotherapeutics due to their ability to simultaneously recognize two different targets. Compared to conventional monoclonal antibodies, knob-into-hole bispecific antibodies face unique challenges in production and characterization due to the increase in variant possibilities, such as homodimerization in covalent and noncovalent forms. In this study, a storage- and pH-sensitive hydrophobic interaction chromatography (HIC) profile change was observed for the hole–hole homodimer, and the multiple HIC peaks were explored and shown to be conformational isomers. We combined traditional analytical methods with hydrogen/deuterium exchange mass spectrometry (HDX MS), native mass spectrometry, and negative-staining electron microscopy to comprehensively characterize the hole–hole homodimer. HDX MS revealed conformational changes at the resolution of a few amino acids overlapping the CH2-CH3 domain interface. Conformational heterogeneity was also assessed by HDX MS isotopic distribution. The hole–hole homodimer was demonstrated to adopt a more homogeneous conformational distribution during storage. This conformational change is likely caused by a lack of CH3 domain dimerization (due to the three “hole” point mutations), resulting in a unique storage- and pH-dependent conformational destabilization and refolding of the hole–hole homodimer Fc. Compared with the hole–hole homodimer under different storage conditions, the bispecific heterodimer, guided by the knob-into-hole assembly, proved to be a stable conformation with homogeneous distribution, confirming its high quality as a desired therapeutic. Functional studies by antigen binding and neonatal Fc receptor (FcRn) binding correlated very well with the structural characterization. Comprehensive interpretation of the results has provided a better understanding of both the homodimer variant and the bispecific molecule.
中文翻译:

“Knob-Into-Hole”双特异性抗体中孔-孔同二聚体变体的结构和功能表征
双特异性抗体由于能够同时识别两个不同的靶标,因此具有成为下一代生物治疗药物的巨大潜力。与传统的单克隆抗体相比,由于变异可能性的增加,例如共价和非共价形式的同二聚化,旋钮入孔双特异性抗体在生产和表征方面面临着独特的挑战。在本研究中,观察到孔-孔同型二聚体的储存和 pH 敏感的疏水相互作用色谱 (HIC) 谱变化,并且探索了多个 HIC 峰并显示为构象异构体。我们将传统分析方法与氢/氘交换质谱(HDX MS)、天然质谱和负染色电子显微镜相结合,全面表征了空穴-空穴同二聚体。HDX MS 揭示了与 C H 2-C H 3 结构域界面重叠的几个氨基酸的分辨率的构象变化。构象异质性也通过 HDX MS 同位素分布进行评估。孔-孔同型二聚体被证明在储存过程中采用更均匀的构象分布。这种构象变化可能是由于缺乏 CH 3 结构域二聚化(由于三个“孔”点突变)引起的,导致孔-孔同型二聚体 Fc 出现独特的储存和 pH 依赖性构象不稳定和重折叠。与不同储存条件下的孔-孔同二聚体相比,由旋钮-孔组装引导的双特异性异二聚体被证明是具有均匀分布的稳定构象,证实了其作为所需治疗的高质量。抗原结合和新生儿 Fc 受体 (FcRn) 结合的功能研究与结构表征密切相关。对结果的综合解释提供了对同二聚体变体和双特异性分子的更好理解。
更新日期:2017-12-01
中文翻译:

“Knob-Into-Hole”双特异性抗体中孔-孔同二聚体变体的结构和功能表征
双特异性抗体由于能够同时识别两个不同的靶标,因此具有成为下一代生物治疗药物的巨大潜力。与传统的单克隆抗体相比,由于变异可能性的增加,例如共价和非共价形式的同二聚化,旋钮入孔双特异性抗体在生产和表征方面面临着独特的挑战。在本研究中,观察到孔-孔同型二聚体的储存和 pH 敏感的疏水相互作用色谱 (HIC) 谱变化,并且探索了多个 HIC 峰并显示为构象异构体。我们将传统分析方法与氢/氘交换质谱(HDX MS)、天然质谱和负染色电子显微镜相结合,全面表征了空穴-空穴同二聚体。HDX MS 揭示了与 C H 2-C H 3 结构域界面重叠的几个氨基酸的分辨率的构象变化。构象异质性也通过 HDX MS 同位素分布进行评估。孔-孔同型二聚体被证明在储存过程中采用更均匀的构象分布。这种构象变化可能是由于缺乏 CH 3 结构域二聚化(由于三个“孔”点突变)引起的,导致孔-孔同型二聚体 Fc 出现独特的储存和 pH 依赖性构象不稳定和重折叠。与不同储存条件下的孔-孔同二聚体相比,由旋钮-孔组装引导的双特异性异二聚体被证明是具有均匀分布的稳定构象,证实了其作为所需治疗的高质量。抗原结合和新生儿 Fc 受体 (FcRn) 结合的功能研究与结构表征密切相关。对结果的综合解释提供了对同二聚体变体和双特异性分子的更好理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号